Novel sofosbuvir crystal
A technology of crystallization and crystallization, which is applied in the field of new crystals for the treatment of hepatitis C compounds, can solve problems such as difficulty in obtaining, polymorphism, curative effect, bioavailability melting point, solubility, stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
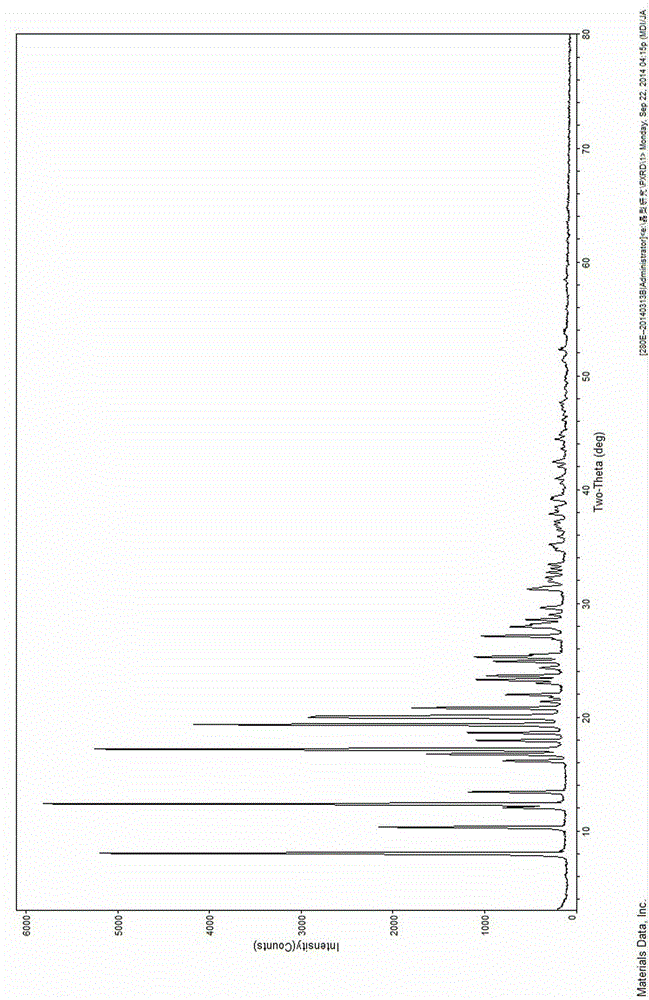
[0036] Take 500mg of sofosbuvir in a round bottom flask of appropriate volume, add 30mL of ethanol, stir and dissolve at the reflux temperature of 50°C, filter while hot to remove impurities, add 50ml of cyclohexane, seal the cloudy liquid and place it at 20°C After static crystallization, the solid was filtered out and dried to obtain 446 mg of sofosbuvir new crystals with a yield of 89.2% and a purity of 99.9%.
[0037] Gained sofosbuvir new crystal has X-ray powder diffraction pattern data as shown below:
[0038]
Embodiment 2
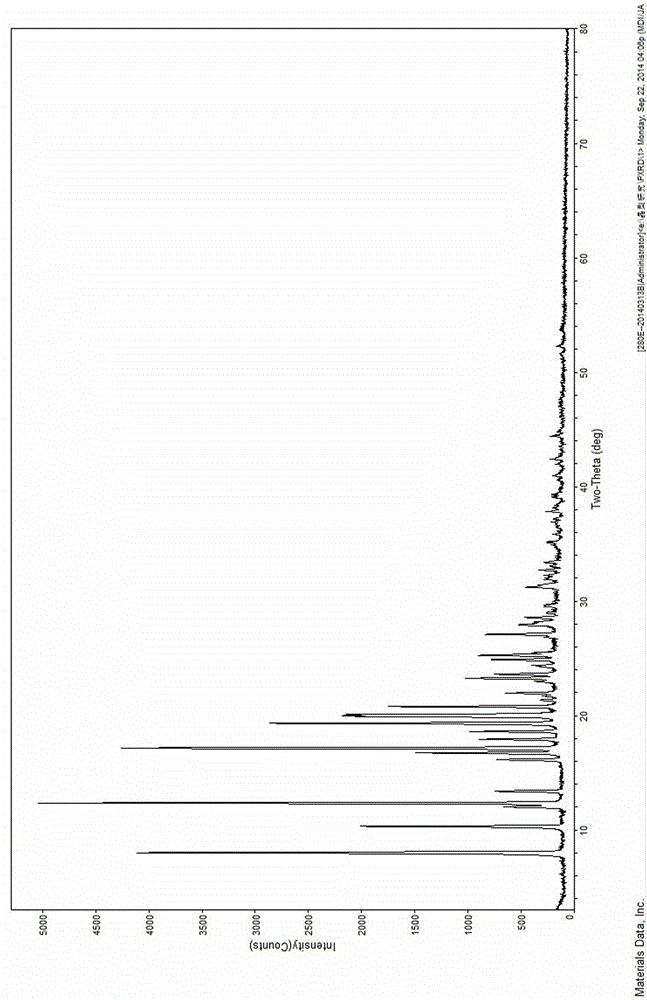
[0040] Take 500mg of sofosbuvir in a round bottom flask of appropriate volume, add 15mL of acetone, stir and dissolve at 45°C, filter while hot to remove impurities, add 30ml of cyclohexane, seal the turbid liquid and place it at 25°C Crystallize, filter and dry the solid to obtain 426mg of sofosbuvir new crystals, with a yield of 85.2% and a purity of 99.7%. The X-ray powder diffraction pattern data are the same as in Example 1.
Embodiment 3
[0042]Take 500 mg of sofosbuvir in a round bottom flask of appropriate volume, add 30 mL of acetone, stir and dissolve at 40 ° C, filter while hot to remove impurities, and add 30 ml of petroleum ether, seal the turbid liquid and place it at 30 ° C for static analysis crystal, filtered out the solid and dried to obtain 430 mg of sofosbuvir new crystal, with a yield of 86.0%, a purity of 99.8%, and its X-ray powder diffraction pattern data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com