Preparation method of sofosbuvir
A technology of sofosbuvir and methyl, which is applied in the field of preparation of sofosbuvir, can solve the problems of difficult raw materials, complex docking or hydrolysis process, etc., and achieve the effect of easy raw materials, low cost and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
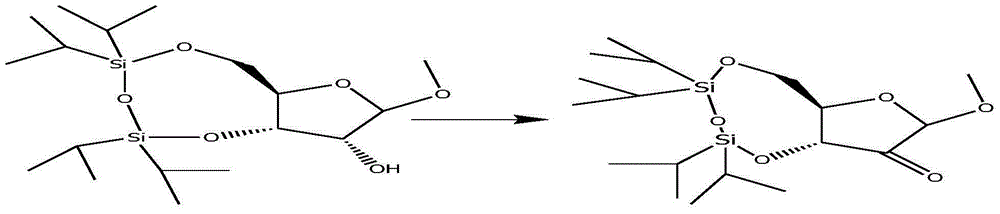
Examples
Embodiment 1
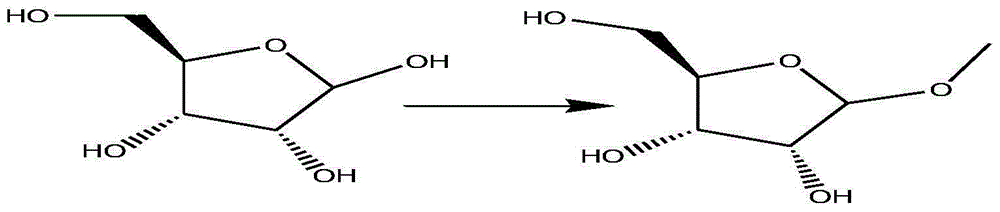
[0028] Add 300ml of anhydrous methanol to a 500ml single-necked bottle, cool to 0-5°C in an ice bath, slowly add 5.2g (20mol%) acetyl chloride dropwise, keep stirring for 20 minutes, add 50g of D-ribose, rise to room temperature and stir After 24 hours, the solvent was removed by concentration to obtain 55 g of white solid product methyl-β-D-nucleoside.
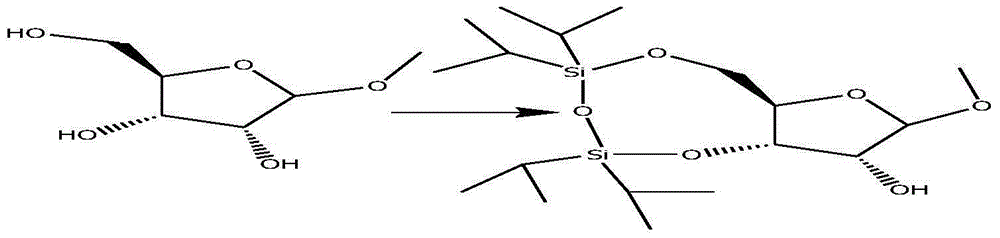
[0029] Add 50 grams of methyl-β-D-nucleoside, 500 milliliters of dichloromethane and 100 milliliters of anhydrous pyridine into a 1-liter reaction flask, cool down to 0°C, slowly add 97.5 milliliters of TIPDSCl dropwise, and use 10 Rinse the dropping funnel with a milliliter of dichloromethane, then rise to room temperature and stir for 3 hours, add 2M hydrochloric acid, separate layers, extract the aqueous layer with 200 ml of dichloromethane, combine the organic phases, wash with saturated brine twice, and dry over sodium sulfate , filtered, and concentrated to obtain 92 grams of the product methyl-3,5-O-(1,1,3,3-tetraisopr...
Embodiment 2
[0037] Add 300ml of anhydrous methanol to a 500ml single-necked bottle, cool to 0-5°C in an ice bath, slowly add 5.2g (20mol%) acetyl chloride dropwise, keep stirring for 30 minutes, add 50g of D-ribose, rise to room temperature and stir After 24 hours, the solvent was removed by concentration to obtain 55 g of white solid product methyl-β-D-nucleoside.
[0038] Add 50 grams of methyl-β-D-nucleoside, 500 milliliters of dichloromethane and 100 milliliters of anhydrous pyridine into a 1-liter reaction flask, cool down to 0°C, slowly add 97.5 milliliters of TIPDSCl dropwise, and use 10 Rinse the dropping funnel with a milliliter of dichloromethane, then rise to room temperature and stir for 3.5 hours, add 2M hydrochloric acid, separate layers, extract the aqueous layer with 200 ml of dichloromethane, combine the organic phases, wash with saturated brine twice, and dry over sodium sulfate , filtered, and concentrated to obtain 92 grams of the product methyl-3,5-O-(1,1,3,3-tetraiso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com