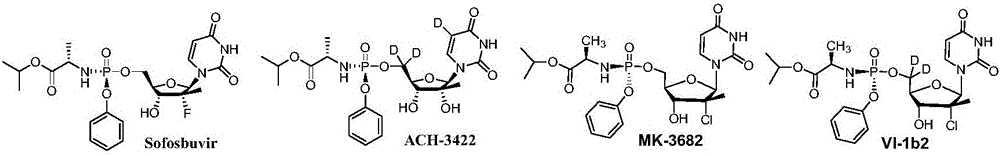
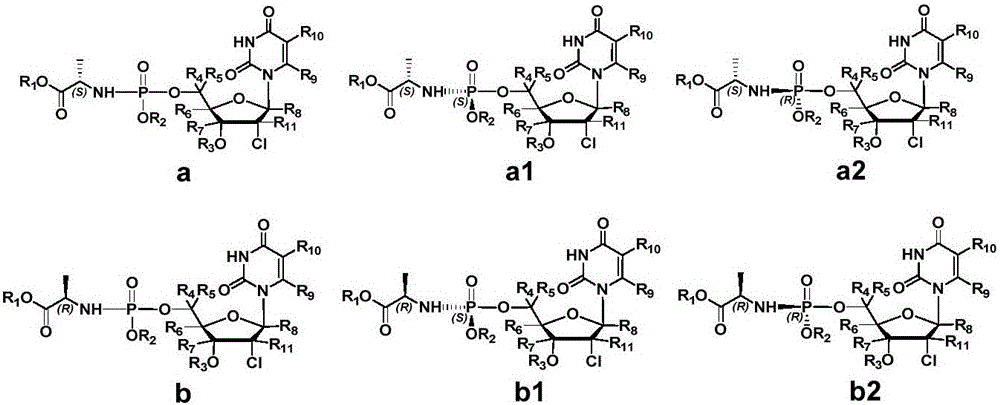
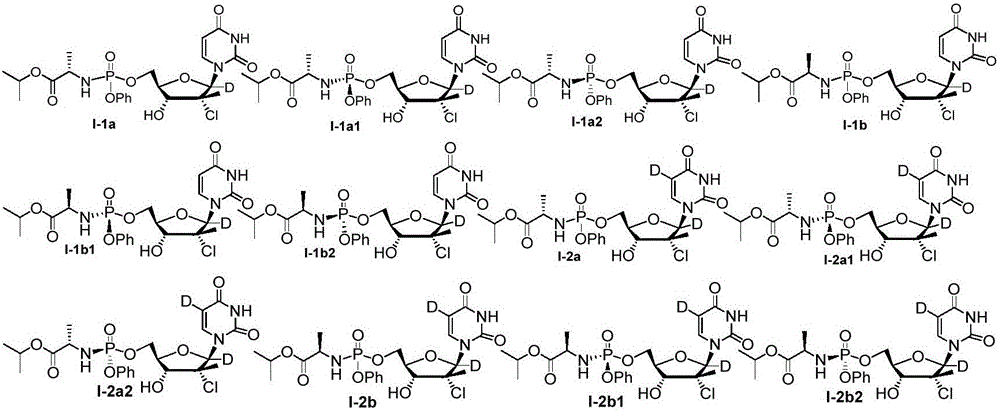
Antiviral nucleoside phosphoramidate and pharmaceutical composition and applications thereof
A technology of nucleoside phosphoramidate and nucleoside derivatives, which is applied in the field of nucleoside phosphoramidate derivatives and their drug combinations, which can solve the problems of rapid drug resistance of hepatitis C, low cure rate, and long treatment course
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Isopropyl[(S)‐(pentafluorophenoxy)(phenoxy)phosphoryl]-L-alaninate (3) and isopropyl[(R)‐(pentafluorophenoxy)( Phenoxy)phosphoryl]-D-alanine ester (6)
[0088]
[0089] Add phenyl dichlorophosphate (5.7 g, 25.7 mmol) and L-alanine isopropyl hydrochloride (4.32 g, 25.7 mmol) into the reaction flask, cool to -70°C within 1.5 hours A solution of triethylamine (7.2 ml, 51.7 mmol) in dichloromethane (22 ml) was added dropwise. After the addition was complete, it was raised to room temperature, and after stirring overnight, the reaction mixture was cooled to 0° C., and pentafluorophenol was dissolved within 40 minutes. (4.73 g, 25.7 mmol) and triethylamine (3.6 ml, 25.7 mmol) in dichloromethane (30 mL) were added dropwise to the above solution, stirred at 0° C. for 1 hour, warmed to room temperature, and stirred overnight, Remove the solid triethylamine hydrochloride by filtration, wash the solid filter cake with dichloromethane (3X10mL), concentrate the filtrate under re...
Embodiment 2
[0093]
[0094] 2.1. N-(tert-butoxycarbonyl)-D-alanine (8a)
[0095] Suspend 4.5g (50mmol) D‐alanine, 3.8g (55mmol), potassium hydroxide and 13.0g (55mmol), di-tert-butyl carbonate in a mixed solvent of water (200mL) and THF (20mL), room temperature Stir overnight to obtain N-Boc-D-alanine white solid 9.6g, which can be directly used in the next step of esterification without purification.
[0096] 2.2. N-(tert-butoxycarbonyl)-D-alanine-deuterated isopropyl ester-d 6 (9a)
[0097] The aforementioned N‐Boc‐D‐alanine 8a (2.18g, 11.5mmol) was dissolved in 40 mL of dry dichloromethane, followed by the addition of hexadeuterioisopropanol‐d 6 (Refer to CN 102010384 and prepare by reduction of deuterated acetone, 15mmol). After cooling the reaction solution to 5°C, EDC (3.31g, 17.2mmol) and DMAP (140mg, 1.15mmol) were added, followed by stirring at room temperature overnight. 300 mL of ethyl acetate was added to dilute the reaction solution, the organic phase was washed with s...
Embodiment 3
[0104] Using the same method as 11a, deuterated alanine esters 9b, 9c and pentafluorophenol were reacted with phenoxyphosphoryl dichloride to prepare deuterated (pentafluorophenoxy)(phenoxy)phosphoryl in one step )-alanine isopropyl ester 11b and 11c:
[0105]
[0106] Compound 11b is identical to 11c NMR spectrum:
[0107] 1 H NMR (400MHz, CDCl 3 )δ(ppm):7.40–7.35(m,2H),7.33–7.20(m,3H),3.95(brs,1H),3.65–3.60(m,1H),1.43(s,6H),1.41(d ,J=7.0Hz,3H). 31 P NMR (162MHz, CDCl 3 )δ‐1.99.
[0108] Compound 11b' is identical to 11c' NMR spectrum:
[0109] 1 H NMR (400MHz, CDCl 3 )δ(ppm):7.42–7.34(m,2H),7.32–7.19(m,3H),3.89(brs,1H),3.66–3.61(m,1H),1.45(s,6H),1.42(d ,J=7.0Hz,3H). 31 P NMR (162MHz, CDCl 3 )δ‐1.63.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com