Synthetic method of sofosbuvir intermediate
A synthesis method and compound technology, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve problems such as large waste water, and achieve the effects of simple industrial operation, reducing the loss of dissolution in water, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
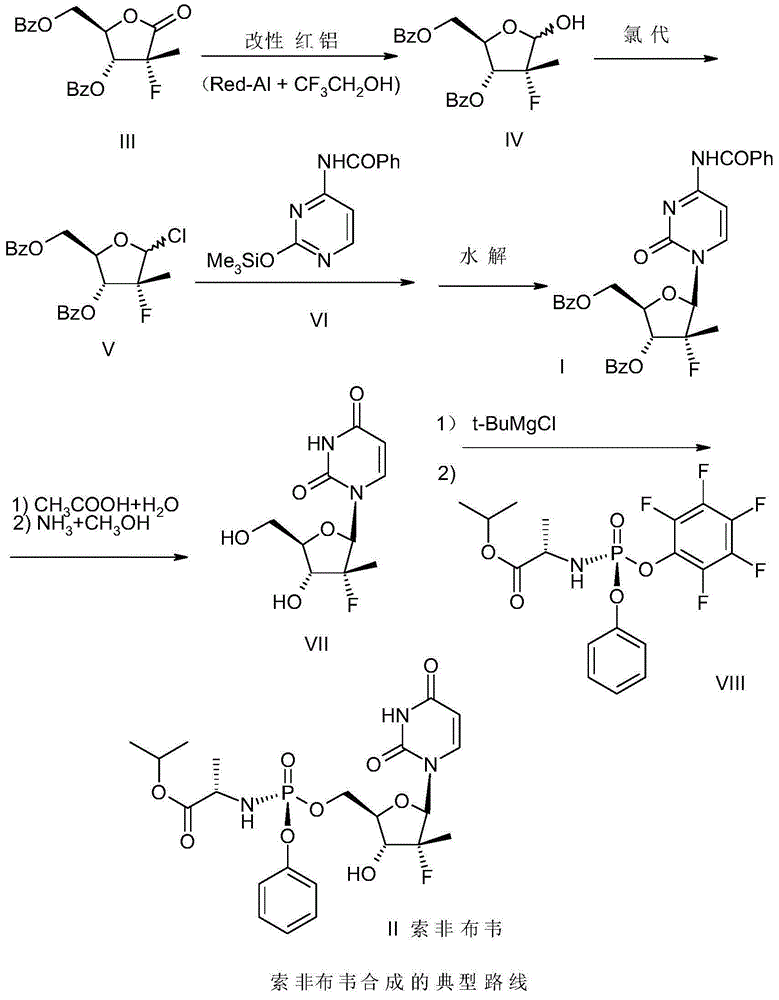
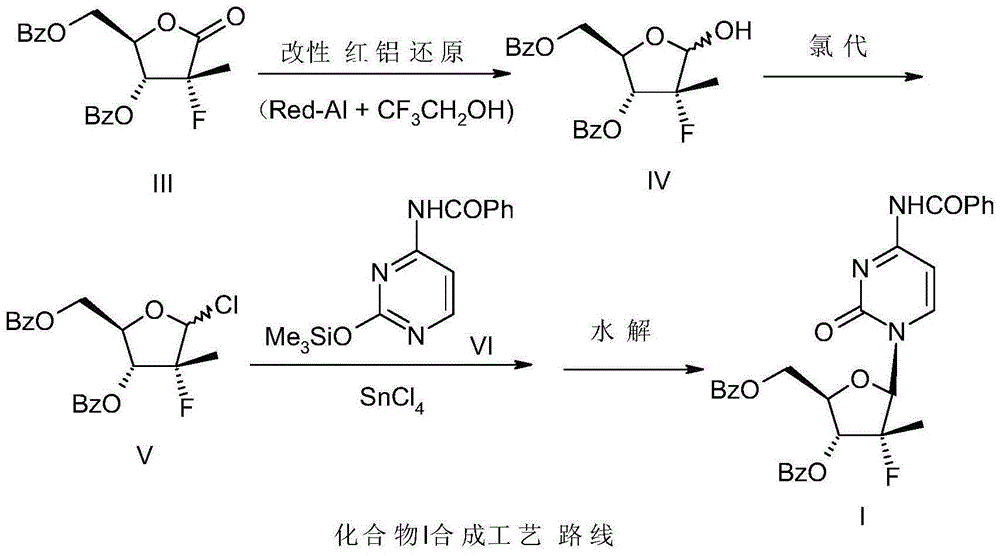
[0026] Example 1 Preparation of (2'R)-N-benzoyl-2'-deoxy-2'-fluoro-2'-methylcytidine-3',5'-dibenzoate
[0027] -25~-15 ℃ temperature, under the protection of nitrogen, 100 grams of red aluminum (70% content), drop into the mixed solution of 35 grams of toluene and 35 grams of trifluoroethanol, after dropping, warm up to room temperature and stir for 1 hour, set aside .
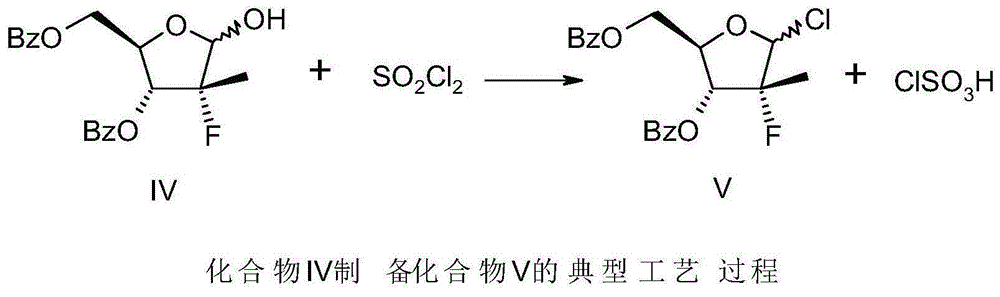
[0028] -20~-15 ℃ temperature, under nitrogen protection, 74.4 grams (0.2mol) 3,5-dibenzoyl-2-deoxy-2-fluoro-2 methyl-D-ribose-γ-lactone (compound III), 150 grams of toluene was added in the reaction flask, slowly dripped into 148 grams of the modified red aluminum solution of the previous step, dripped in about 3 hours, and kept warm for 30 minutes after the dripping, TLC tracked the disappearance of the raw material point, and obtained compound IV Toluene solution.
[0029] To the reaction solution in the previous step, add 2.9 g (0.04 mol) of N,N-dimethylformamide (DMF) at a temperature of -20 to -15 ° C, ...
Embodiment 2~12
[0034] Referring to Example 1, different SOCl 2 , DMF consumption, the reaction yield when thionyl chloride dripping temperature is different, chlorination temperature is different (except chlorination process, feed intake and other treatment methods are identical with embodiment 1), reaction condition and result are as shown in table 1 below :
[0035] Table 1
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com