An improved sofosbuvir preparation method
A compound, Lewis acid technology, applied in the field of medicine and chemical industry, can solve problems such as unsafe
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
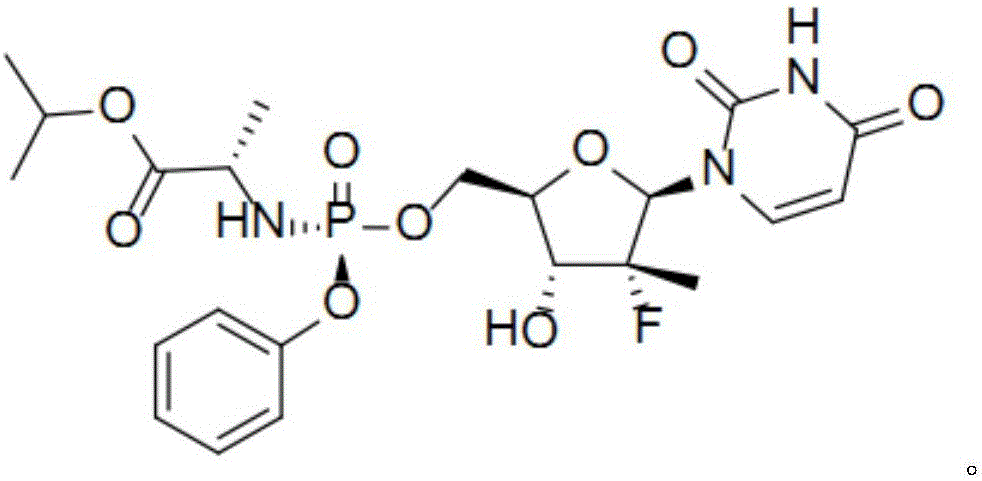
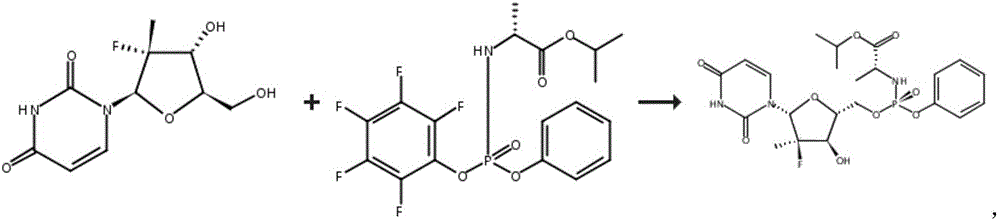
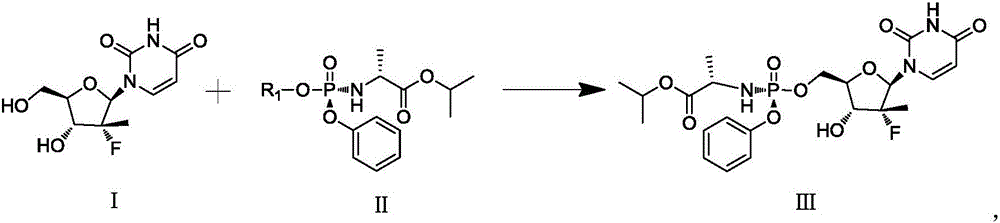
[0034] Specifically, the improved sofosbuvir preparation method of the present invention may include:
[0035] a) adding compound I, compound II, Lewis acid and organic solvent into the reaction vessel, and adding alkali;
[0036] b) Control the reaction temperature of the system at 20°C to 40°C, and stir the reaction until the compound I content is ≤3.0%;
[0037] c) stop the reaction, extract the reaction solution, and then perform crystallization to obtain compound III.
[0038] Specifically, the method for preparing the improved sofosbuvir described in the present invention may include the following steps:
[0039] a) adding compound I, compound II, Lewis acid and organic solvent into the reaction vessel, and adding alkali;
[0040] b) Control the reaction temperature of the system at 20°C to 40°C, and stir the reaction until the compound I content is ≤3.0%;
[0041] c) Stop the reaction, extract the reaction solution with dichloromethane, wash with aqueous sodium carbo...
specific Embodiment approach
[0081] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0082] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0083] In the present invention, ml or mL refers to milliliter, L refers to liter, g refers to gram, kg refers to kilogram, mol / L refers to mole / liter, h refers to hour, min refers to minute, v / v refers to volume ratio, <a certain value is means below a certain value.
[0084] HPLC detection chromatographic conditions are as follows:
[0085] Chromatographic column: Waters XSELECT HSS T3 (4.6*100mm, 2.5um);
[0086] Flow rate: 1.5mL / min; Detection wavelength: 210nm; Column temperature: 25°C;
[0087] Running time: 29min; Injection volume: 2μl;
[0088] Mobile phase: Phase A: Dissolve 0.5mL of ...
Embodiment 1
[0092] Add 100g of compound Ⅰ, 51g of anhydrous magnesium chloride, 200g of compound Ⅳ, 600mL of tetrahydrofuran into the reaction flask, add 60g of DIPEA dropwise under stirring at a temperature of 30°C to 40°C, after the addition is completed, react at a temperature of 30°C to 40°C, and detect by HPLC Compound I content ≤ 3.0%, stop the reaction. Add 300 mL of 10% (v / v) dilute hydrochloric acid to the reaction solution, stir for 30 min, add 600 g of dichloromethane for extraction, and separate the aqueous phase; the organic phase is washed three times with 140 g of 10% sodium carbonate, and the aqueous sodium carbonate phase is combined , after extracting twice with 100 g of dichloromethane, combine all the dichloromethane phases, wash once with 100 mL of water, evaporate to dryness, then add 50 mL of isopropyl acetate, continue to evaporate to dryness, add 1.2 L of isopropyl acetate to the resulting product, After heating to 60°C to dissolve, cool down to 20°C, stir until s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com