Preparation method for hepatitis C virus resisting drug sofosbuvir intermediates
A technology of sofosbuvir and intermediates, which is applied in the field of preparation of anti-hepatitis C virus drug sofosbuvir intermediates, and can solve the problems of long reaction routes and high costs of compounds 9 and 11
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
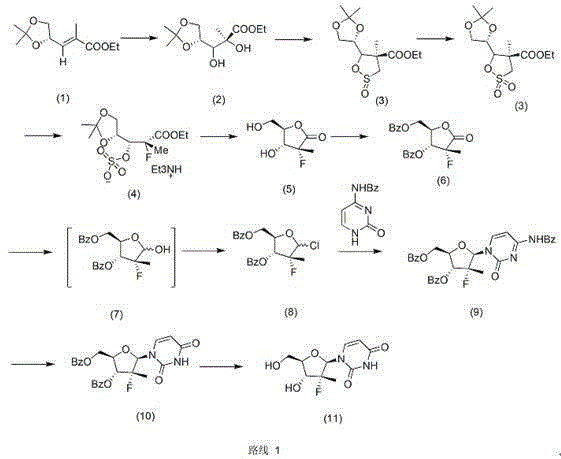
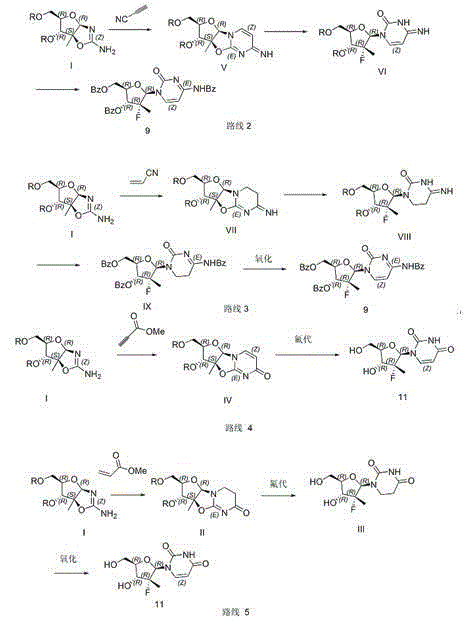
[0012] Example 1 (preparation of compound IV)
[0013] Add 94g of compound I and 42g of methyl propiolate, 250mL of 50% ethanol solvent, 152g of 1,8-diazabicycloundec-7-ene into a 500mL reaction bottle, heat up and reflux for 4 hours, then cool down to room temperature After concentrating the solvent to half, 100 mL of isopropanol was slowly added dropwise to precipitate crystals, filtered, and the filter cake was vacuum-dried for 5 hours to obtain 110 g of a white solid with a yield of 91.7% and a purity of 98%. H-NMR (MeOD400MHz): δ1.64 (3H, s), 3.49-3.54 (1H, dd), 3.58-3.63 (1H, dd), 4.05-4.09 (1H, dd), 4.33 (1H, d), 5.90 (1H, s), 6.05 (1H, d), 7.76 (1H, d).
example 2
[0014] Example 2 (preparation of compound 11)
[0015] Add 80g of compound IV, 19.31g of potassium fluoride, 160mL of DMF, 160mL of toluene, and 1g of benzo-18-crown-6-ether into a 500mL reaction flask. Add 200mL of 1,4-dioxane dropwise, precipitate needle-like crystals, cool to 5-10°C, filter, filter the cake with 200mL of ethyl acetate for 1 hour and filter, vacuum-dry at 45°C for 5 hours to obtain 80g of white needle-like crystals , yield 92.3%, purity 98.5%. H-NMR (DMSO-d 6 ): δ11.44(br, s, 1H, NH), 7.95(d, 1H), 5.97(d, 1H), 5.64(d, 1H), 3.84-3.77(m, 3H), 3.63-3.60(m , 1H), 1.23(d, 3H).
example 3
[0016] Example 3 (preparation of compound V)
[0017] Add 180g of I, 240mL of N,N-dimethylacetamide to a 500mL reaction bottle, control the temperature at 15-20°C and add 40g of propylonitrile, about 1.5 hours, the system turns dark reddish brown, add 50mL of 48% hydrogen fluoride, 10mL of water, After reacting at 20-25°C for 2 hours, 80 mL of 1,4-dioxane was slowly added dropwise, and 85 g of crystalline solid (V) was precipitated, with a yield of 84% and a purity of 97%. H-NMR (DMSO-d 6 ): δ1.63(s, 3H), 1.72(d, 1H), 3.39(dd, 2H), 3.88(s, 1H), 4.53(d, 1H), 4.95(s, 1H), 5.47(s, 1H), 5.71 (s, 1H), 4.95 (s, 1H), 6.55 (m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com