Preparation method of sofosbuvir intermediate
An intermediate and volume ratio technology, applied in the field of intermediate preparation, can solve the problems of cumbersome post-processing operations, low product purity, and low reaction conversion rate, and achieve high reaction conversion rate and product purity, environmental friendliness, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Dissolve 6.23g (29.8mmol) of phenyl dichlorophosphate and 5g (29.8mmol) of L-alanine isopropyl hydrochloride in 50mL DCM under the protection of nitrogen. The dichloromethane solution of the amine (6.01 g of triethylamine dissolved in 5 mL of DCM) was added dropwise into the reaction system, and the temperature was naturally raised to react overnight.
[0049] Add 5.70g (31.0mmol) of pentafluorophenol to the above reaction system, under the same nitrogen protection, control the temperature at about 20°C, add a dichloromethane solution of triethylamine (6.01g of triethylamine is dissolved in 5mL DCM) dropwise, after the addition is complete The reaction takes about 3 hours.
[0050] After the reaction was completed, the reaction solution was poured into ice water, the organic phase was washed twice with saturated sodium bicarbonate (50mL×2), and finally washed once with saturated brine 50mL until neutral, and all the aqueous layers were combined and extracted once with 3...
Embodiment 2
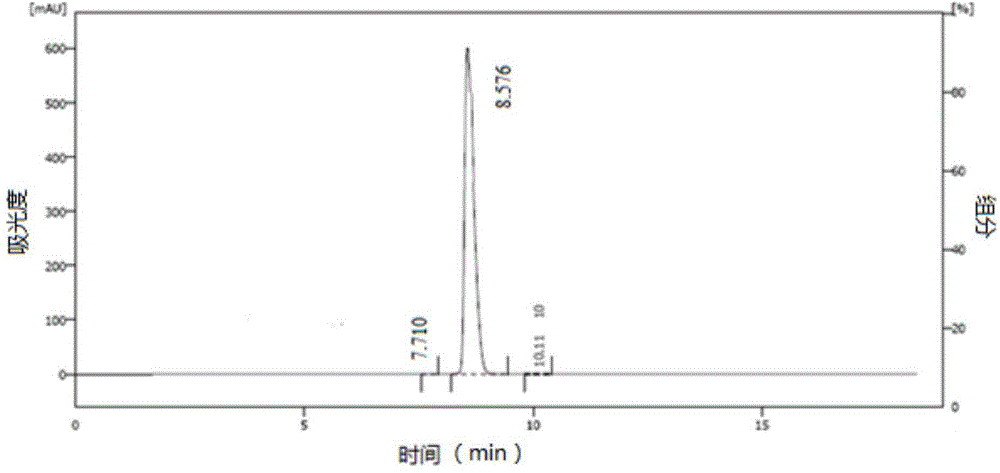
[0061] According to the preparation method of the compound of formula (II) in Example 1, after the compound of formula (II) is obtained, crystallize and purify with n-heptane and ethyl acetate (4:1), and filter to obtain the mother liquor (the isomer in the mother liquor (i.e. the formula III compound) with a mass content of 79%, and the product (compound of formula (I)) with a mass content of 21%), the mother liquor was concentrated to dryness to obtain a mother liquor concentrate, which was refrigerated for use. Take 10 g of mother liquor concentrate, dissolve in 50 mL of dry ethyl acetate, add 0.5 g of triethylamine, stir at room temperature for 10 hours, NH 4 Cl washed and dried. Through HPLC detection (chiral purity), the mass content of isomer is 43%, and the mass content of product is 56% (see image 3 ). Concentrate properly, add n-heptane, precipitate a white solid, and filter. HPLC detection (chiral purity), the mass content of isomer is 1.3%, and the mass content...
Embodiment 3
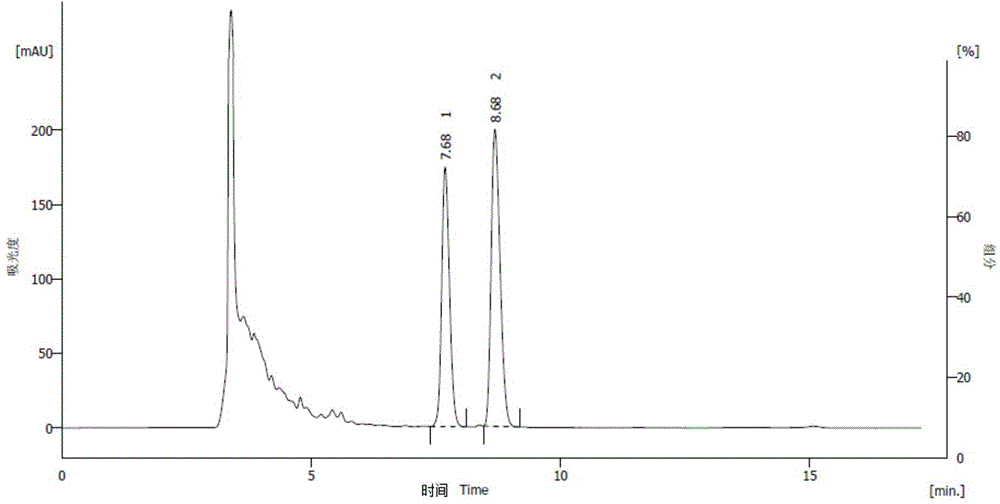
[0071] According to the preparation method of the compound of formula (II) in Example 1, after the compound of formula (II) is obtained, crystallize and purify with n-heptane and ethyl acetate (4:1), and filter to obtain the mother liquor (the isomer in the mother liquor (i.e. the formula III compound) mass content is 79%, product (formula (I) compound) mass content is 21%), mother liquor is concentrated to dryness, stand-by. Take 3 g of mother liquor concentrate, dissolve in 20 mL of dry methyl tert-butyl ether, add 0.1 g of potassium tert-butoxide, stir at room temperature for 12 hours, NH 4 Cl washing, drying, crude product is detected (chiral purity) through HPLC, and the mass content of isomer is 46%, and the mass content of product is 54% (see Figure 5 ). Concentrate properly, add n-heptane, precipitate a white solid, and filter. HPLC detection showed that the mass content of the isomer was 1.0%, the mass content of the product was 99.0%, and the mass of the precipita...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com