Antibody conjugated medicine and preparation method and application thereof
An antibody-conjugated drug and anti-tumor drug technology, applied in the field of biotechnology and medicine, can solve the problems of improving purification difficulty, activity restricted by endocytosis, low homogeneity, etc., avoiding the use of organic reagents, high body Internal and external antitumor activity and homogeneity improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Preparation of OFA-HL Antibody
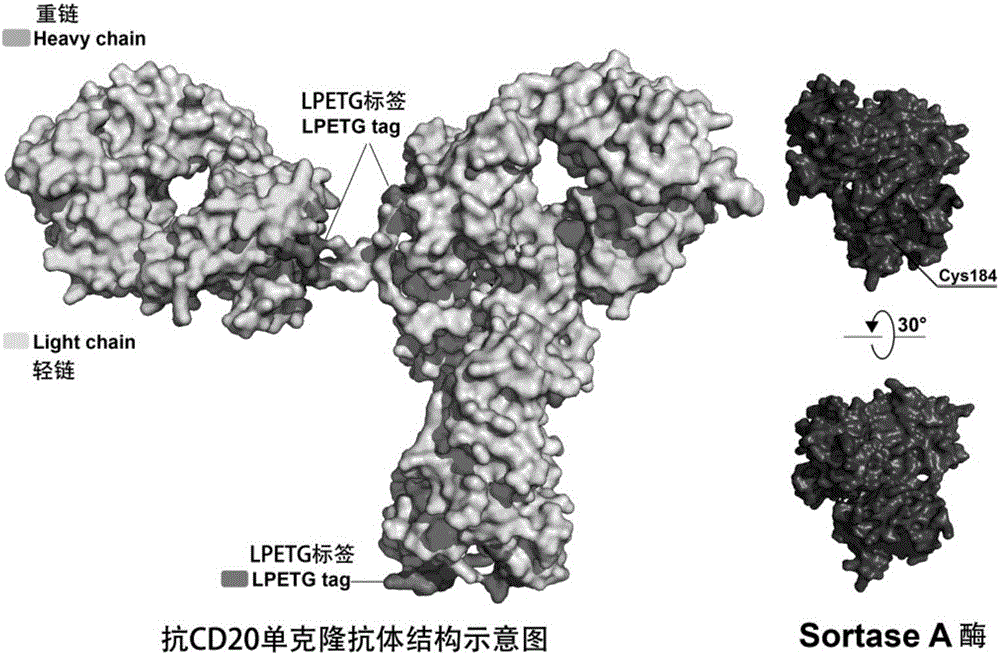
[0071] 1. Construct the expression vector of anti-CD20 monoclonal antibody with LPETG tag on the heavy chain
[0072] A plasmid carrying the anti-CD20 monoclonal antibody gene was constructed using the expression vector of the heavy chain anti-CD20 monoclonal antibody with LPETG (Ofatumumab-HeavyChain-LPETG, hereinafter abbreviated as OFA-HL). For the specific construction method, refer to Chinese invention patent ZL201310046396.9 and ZL201310170344.2.
[0073] Using the above plasmid as a template and P1 and P2 as primers, the expression gene of LPETG was fused to the 3' end of the antibody heavy chain gene by PCR method to obtain the anti-CD20 monoclonal antibody heavy chain gene with LPETG tag. After double digestion, the gene obtained above was subcloned into the original pFUSE-CHIg-hG1 expression vector to obtain the expression vector IgH-OFA-HL expressing OFA-HL heavy chain. The vector for expressing the OFA-HL light ch...
Embodiment 2
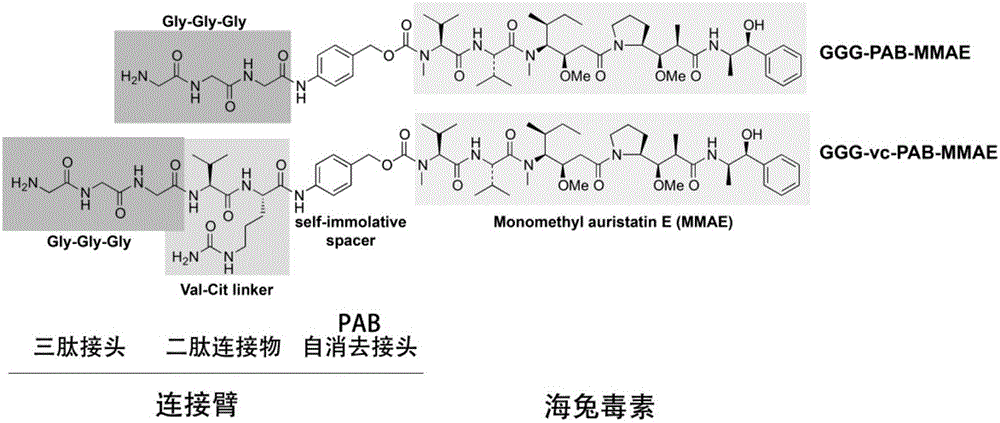
[0089] Example 2 Preparation and further purification and analysis of anti-CD20 monoclonal antibody-dolastatin conjugate
[0090] 1. Expression and purification of Sortase A enzyme and its mutants
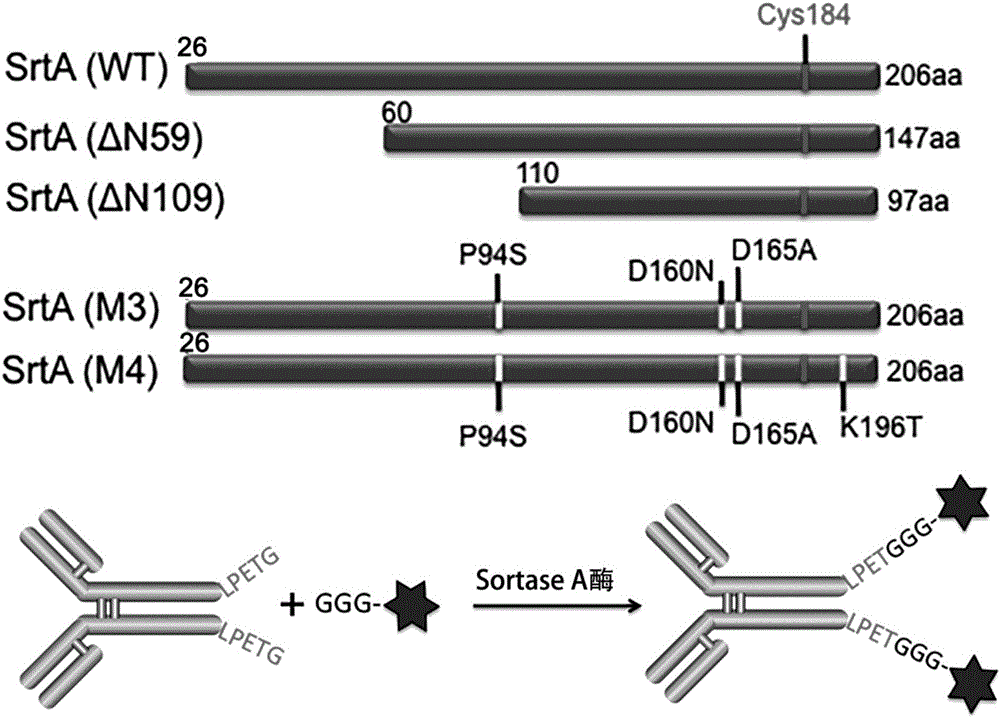
[0091] First, the genome of Staphylococcus aureus was extracted using the Takara Bacterial Genome Extraction Kit, and then the wild-type Sortase A enzymes of different lengths were amplified with the following primers (as shown in Table 1) by PCR. The genes for the different Sortase A enzymes were inserted between the NcoI and XhoI restriction sites on the pET28(+) plasmid.
[0092] After the correct sequence was verified by sequencing, pET28-SrtA(M4) and pET28-SrtA(M3) were constructed using Agilent's point mutation kit (QuikChange IISite-Directed Mutagenesis Kit) using the pET28-SrtA(△N59) expression vector as a template Expression vector, the primers used are as follows (as shown in Table 1).
[0093] Schematic diagram of the length and mutation of Sortase A enzymes of differe...
Embodiment 3
[0130] Example 3 Study on the Affinity of ADC Prepared by Sortase A Enzyme and CD20+ Tumor Cells
[0131] The OFA, OFA-HL, OFA-HL-MMAE and OFA-HL-vcMMAE of each concentration gradient were mixed with ice-cold incubation buffer (10% BSA in PBS, pH 7.4) containing 106 Ramos cells, and placed on ice Place on ice for 30 min; wash twice with pre-cooled PBS (pH 7.4), then add FITC-labeled goat anti-human secondary antibody diluted in PBS containing 1% BSA, and incubate on ice for 30 min; after washing twice, use flow cytometer Cytomics FC 500MCL (Beckman Coulter) detects mean optical density (MFI). The sample concentration is taken as the abscissa, and the corresponding MFI is drawn as the ordinate.
[0132] attached Figure 14 is the affinity of the anti-CD20 monoclonal antibody OFA and its conjugates to Ramos cells. attached Figure 14 It can be seen that adding an LPETG tag to the C-terminus of OFA's heavy chain will slightly reduce its affinity for Ramos cells, and using Sor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com