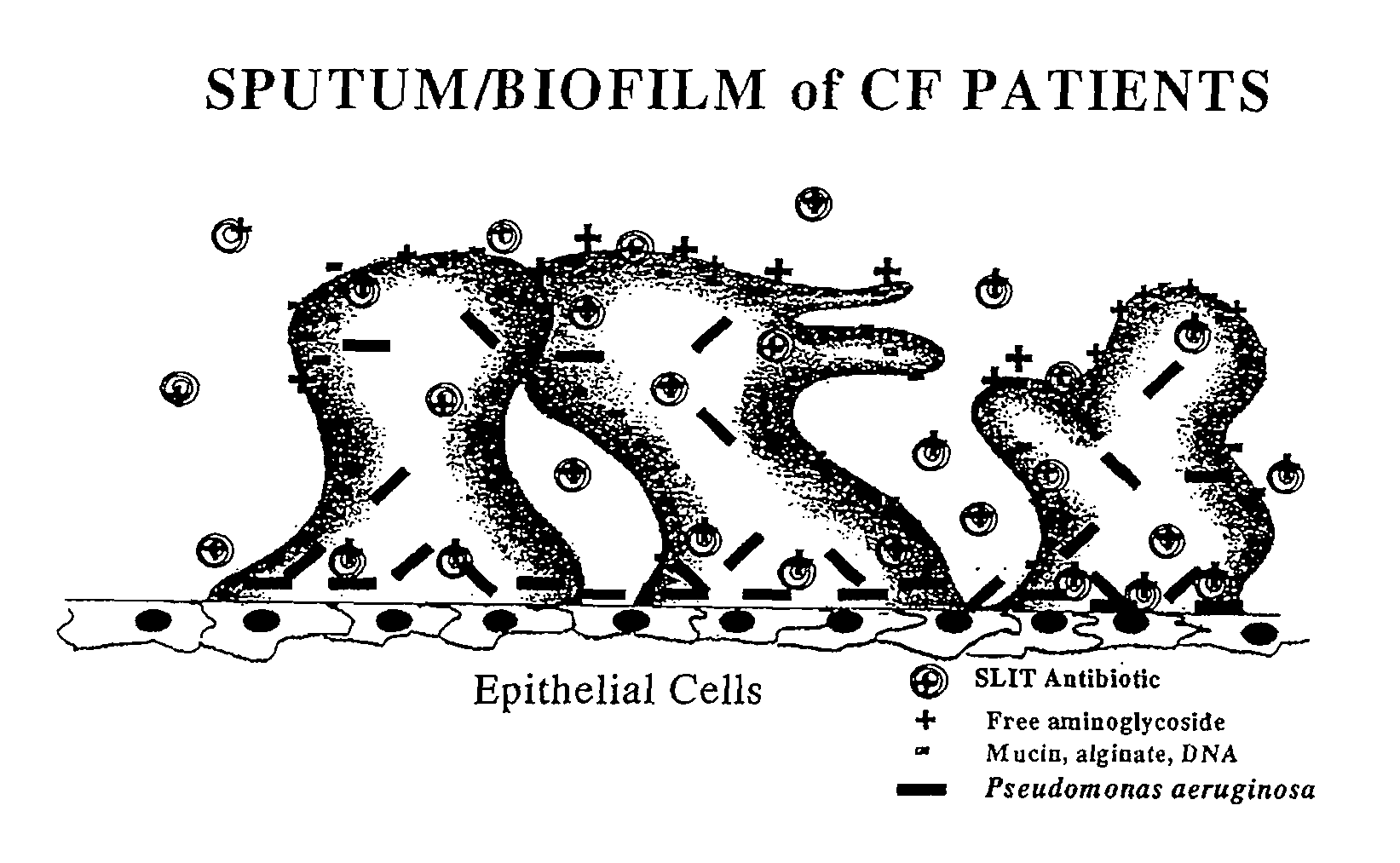
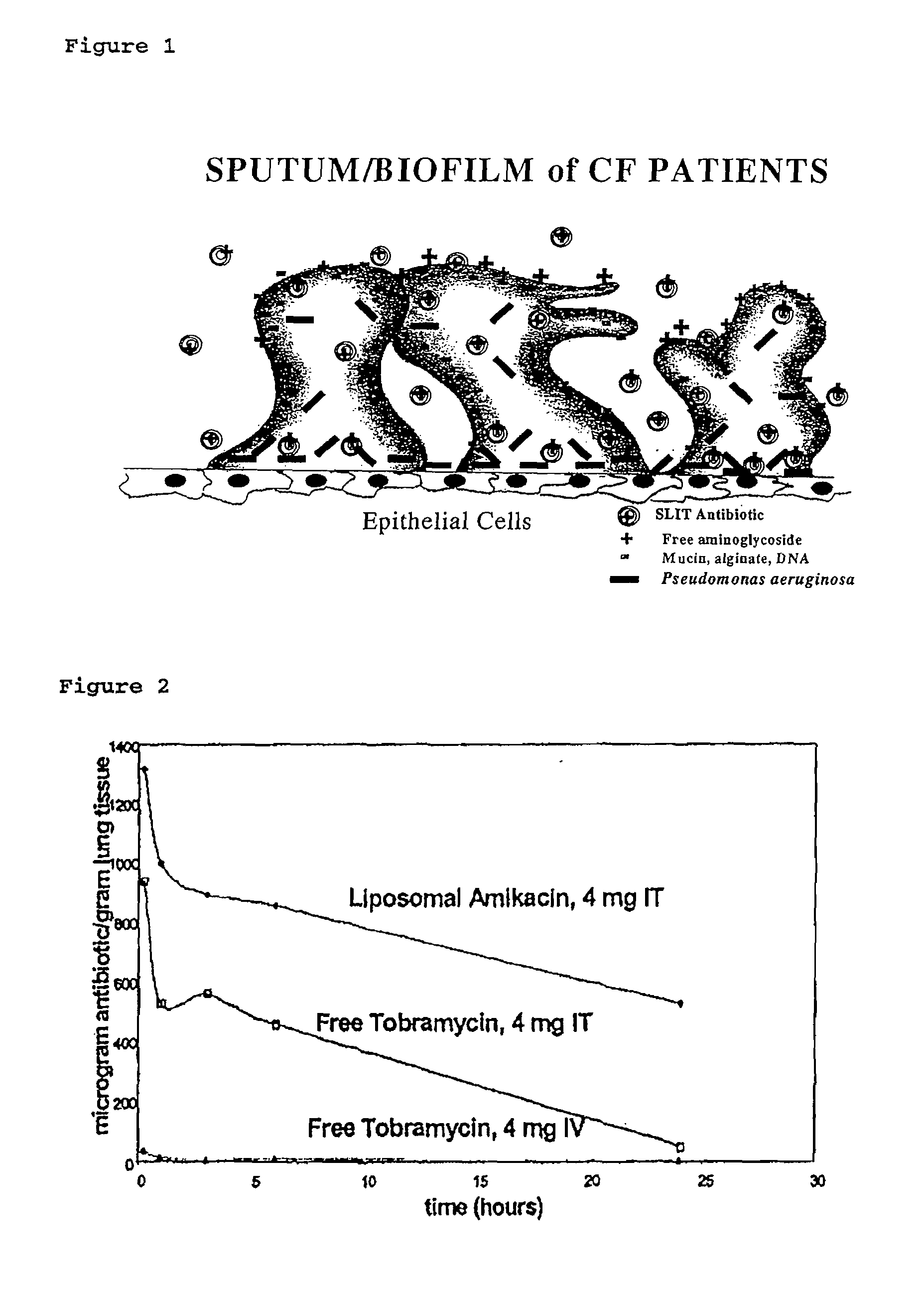
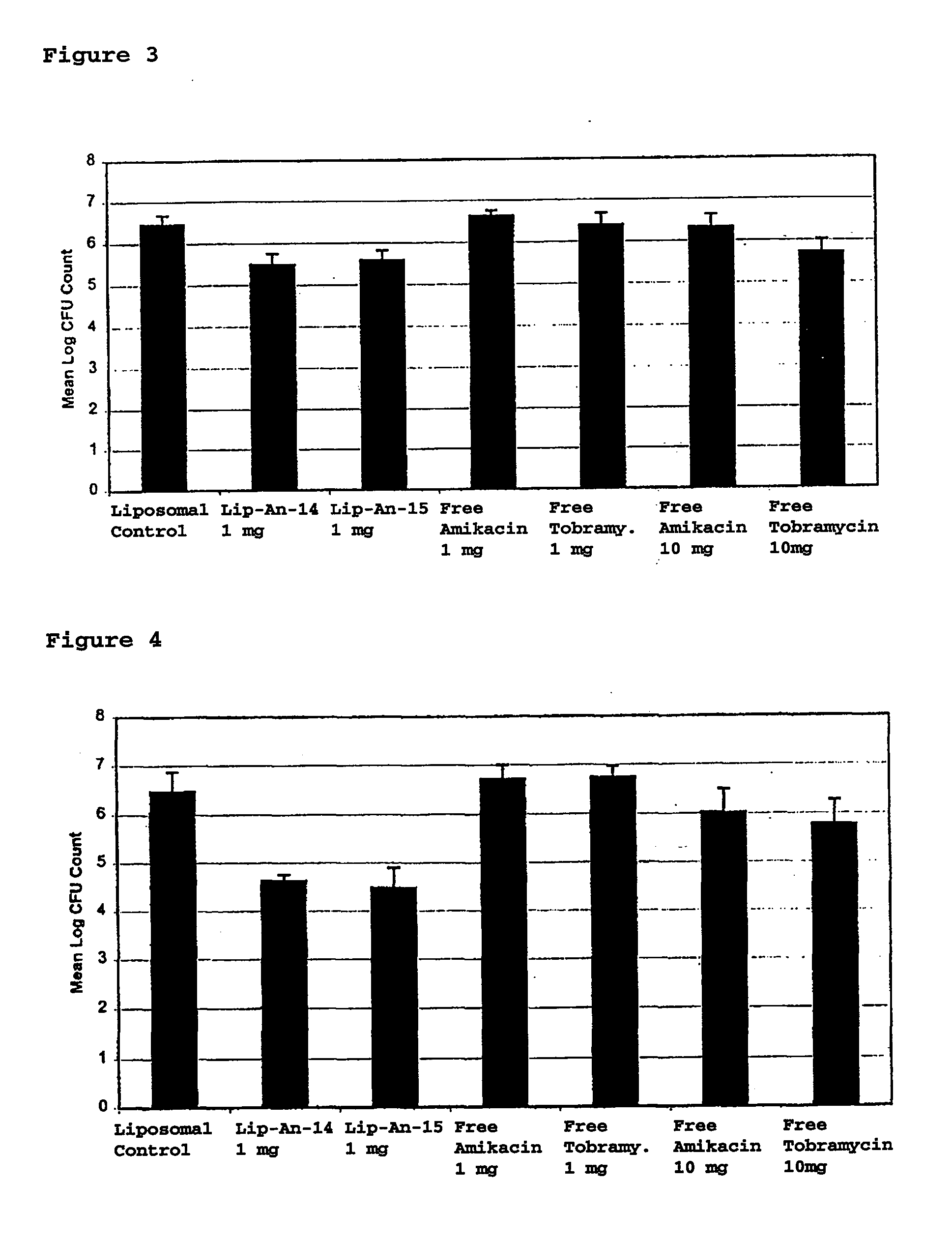
Sustained Release of Antiinfectives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0075]The following is a detailed description of the manufacture of 150 mL of Liposomal / complexed amikacin.[0076]Total Initial Volume=1.5 L[0077]Ethanol Content=23.5% (v / v)[0078]Lipid Composition: DPPC / Chol (1:1 mole ratio)[0079]Initial [Lipid]=7.6 mg / ml[0080]Initial [amikacin sulfate]=57.3 mg / ml[0081]Final product Volume=150 mL
[0082]I) Compounding and Infusion:
[0083]7.47 g DPPC and 3.93 g Cholesterol were dissolved directly in 352.5 mL ethanol in a 50 C water bath. 85.95 g amikacin sulfate was dissolved directly in 1147.5 mL PBS buffer. The solution is then titrated with 10N NaOH or KOH to bring the pH to approximately 6.8.
[0084]352.5 mL ethanol / lipid was added or infused to the 1147.5 mL amikacin / buffer to give a total initial volume of 1.5 L. The ethanol / lipid was pumped @ 30 mL / min (also called infusion rate) with a peristaltic pump into the amikacin / buffer solution which was being rapidly stirred at 150 RPM in a reaction vessel on a stir plate at room temperature
[0085]The produ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com