Preparation method and application of acid-responsive polymer drug based on dextran
A technology of dextran and polymers, which is applied in the fields of chemical drugs and biological applications, and can solve the problems of poor selective and controllable release of drugs, low drug loading, and poor stability of micelles.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
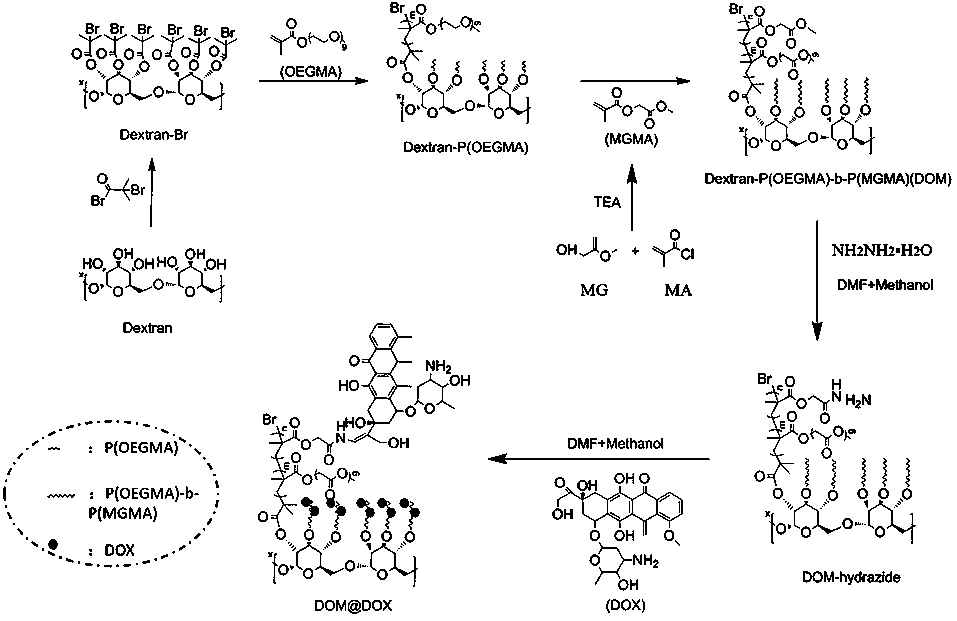
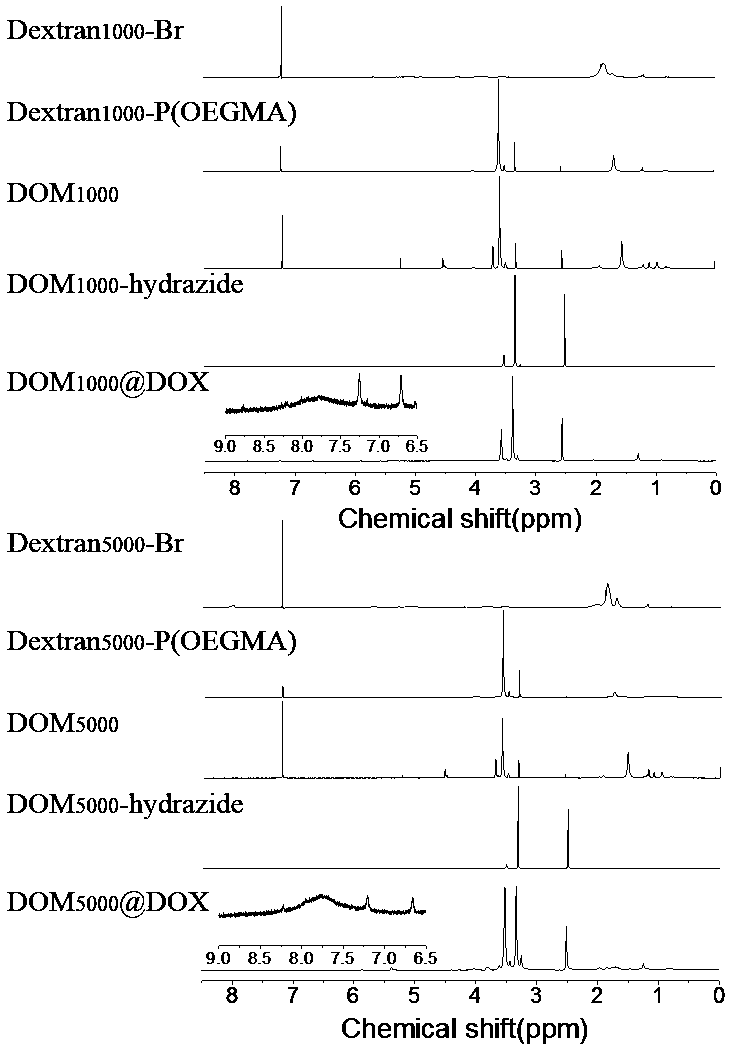
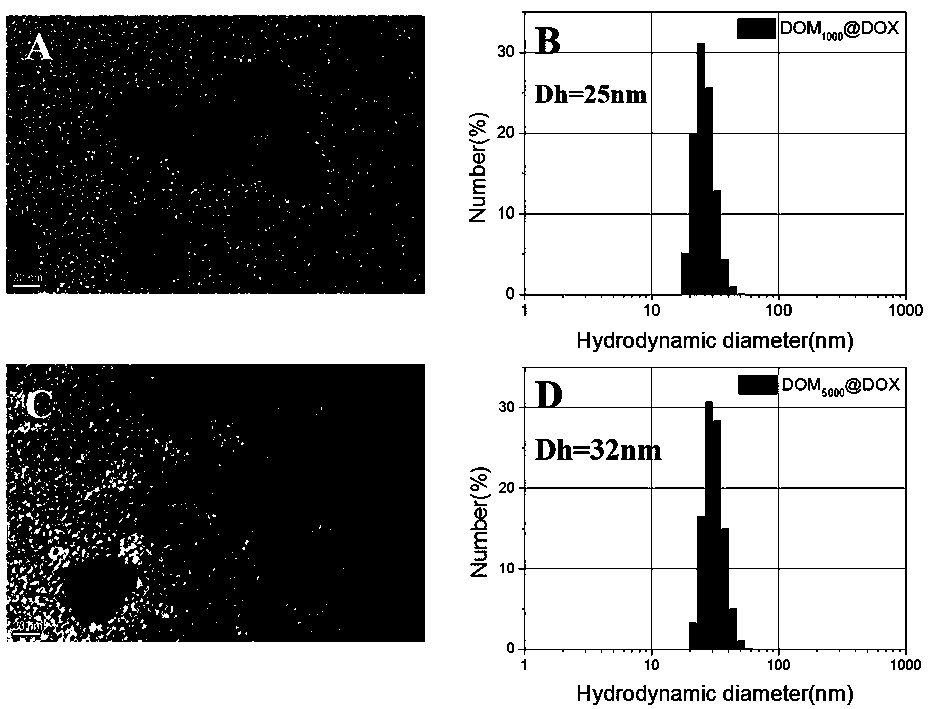
[0048] Example 1 Preparation of dextran-based acid-responsive polymer drug Dextran-P(OEGMA)-b-P(MGMA-DOX)
[0049] (1) Preparation of dextran-based initiator Dextran-Br:
[0050] Under the condition of argon atmosphere, dextran (Dextran, 5g) dissolved in ionic liquid (25.0g) was cooled to 0°C, and N-methylpyrrolidone (NMP, 20mL) and N,N-dimethyl 2-bromoisobutyryl bromide (BIBB, 168.54mmol, 21mL) was slowly added dropwise, and the ice bath was continued for 0.5-2h, then warmed to room temperature (25°C), protected from light Reaction for 12-72h; b) after the reaction, the resulting mixed solution was added dropwise to deionized water to stir and precipitate, and the obtained precipitate was dissolved with acetone and reprecipitated. This was repeated three times to obtain a light yellow intermediate product, which was dried in a vacuum oven (25 -30°C); c) Dissolve the above light yellow intermediate product (500mg) in NMP (5mL), cool in an ice bath to 0°C, slowly add BIBB (5g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com