Lu AE58054 hydrochloride crystal form A, preparation method and uses thereof
A hydrochloride and crystal form technology, applied in the hydrochloride crystal form A of Lu AE58054 and its preparation and use fields, can solve problems such as unsatisfactory solubility, and avoid changes in bioavailability and efficacy, solubility High and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The preparation method of LuAE58054 hydrochloride crystal form A:
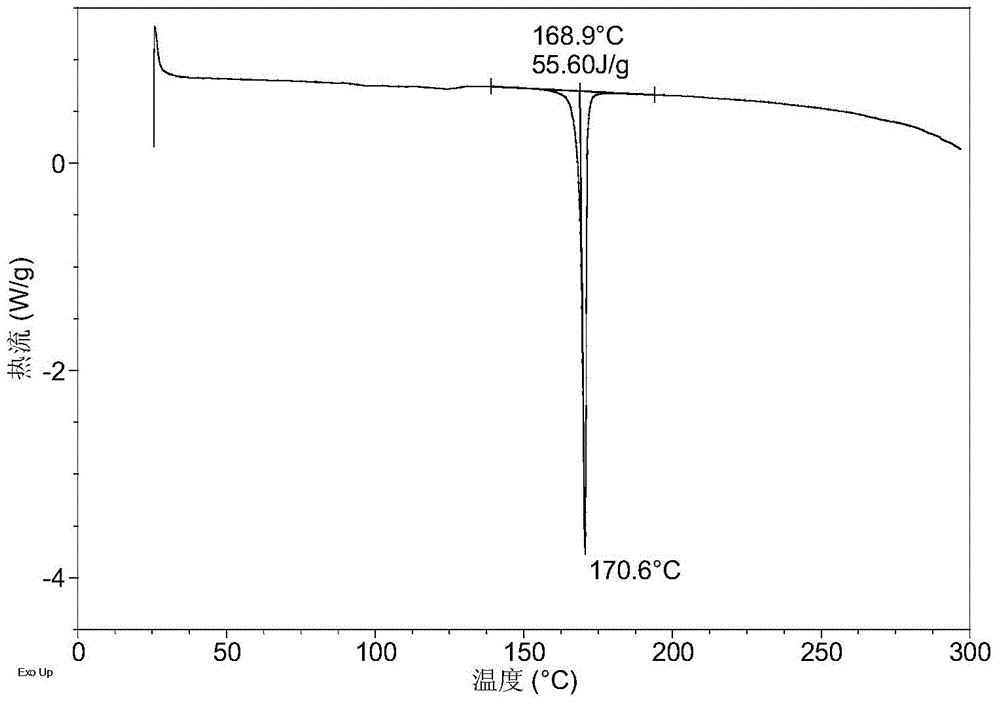
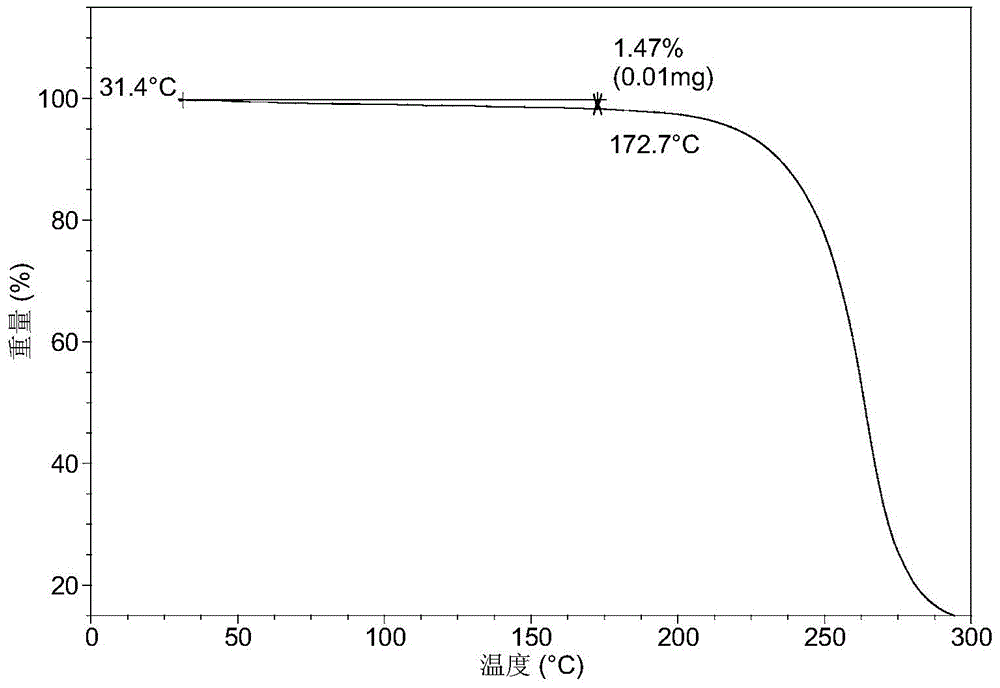
[0072] 10.1 mg of solid LUAE58054 hydrochloride was dissolved in 0.05 mL of ethanol to give a clear solution. With stirring, 0.4 mL of pure water was added dropwise until turbidity appeared, and stirring was continued for 48 hours at room temperature. The solid was collected to obtain Form A of LUAE58054 hydrochloride.
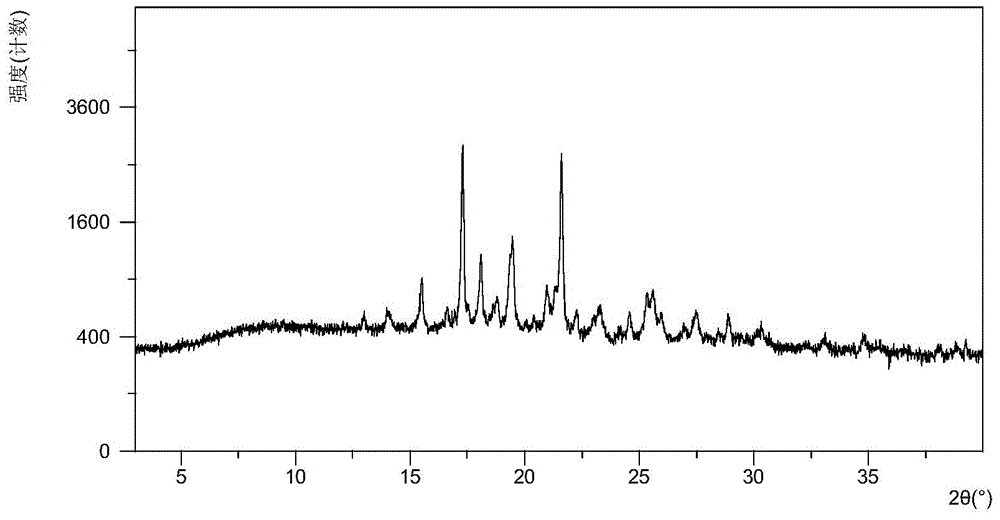
[0073] The X-ray powder diffraction data of the obtained hydrochloride crystal form A are shown in Table 1, and the XRPD figure is as follows figure 1 shown. Considering many factors such as d value, low angle, intensity, characteristic line and peak shape integrity, the diffraction peaks at 2theta values of 17.05°, 17.85°, and 21.37° are characteristic peaks. The diffraction peaks at 2theta values of 15.26°, 19.22°, and 20.73° are important peaks. The diffraction peaks at 2theta values of 12.76°, 27.2, and 25.38° are the next most important peaks.
[0074] Table 1 X-ray powd...
Embodiment 2
[0081] The preparation method of LuAE58054 hydrochloride crystal form A:
[0082] 9.5 mg of solid LuAE58054 hydrochloride was dissolved in 0.025 mL of tetrahydrofuran to give a clear solution. With stirring, 0.3 mL of n-heptane was added dropwise until cloudiness appeared, and stirring was continued for 48 hours at room temperature. The solid was collected to obtain Form A of LuAE58054 hydrochloride.
[0083] Table 2 shows the X-ray powder diffraction data of the hydrochloride crystal form A obtained in this example. Considering many factors such as d value, low angle, intensity, characteristic line and peak shape integrity, the diffraction peaks at 2theta values of 17.03°, 17.87°, and 21.35° are characteristic peaks. The diffraction peaks at 2theta values of 15.27°, 19.24°, and 20.77° are important peaks. The diffraction peaks at 2theta values of 12.72°, 27.30°, and 25.41° are the next most important peaks.
[0084] Table 2 X-ray powder diffraction data of crystal f...
Embodiment 3
[0088] The preparation method of LuAE58054 hydrochloride crystal form A:
[0089] 10.0 mg of LuAE58054 hydrochloride solid was dissolved in 0.6 mL of tetrahydrofuran: water = 2:25 (v:v, volume ratio) mixed solvent, and placed at 50° C. for 30 minutes. The above solution was filtered, and the resulting filtrate was immediately placed in a thermostat at 5° C. and stirred for 48 hours. The solid was collected to obtain Form A of LuAE58054 hydrochloride.
[0090] Table 3 shows the X-ray powder diffraction data of the hydrochloride crystal form A obtained in this example. Considering many factors such as d value, low angle, intensity, characteristic line and peak shape integrity, the diffraction peaks at 2theta values of 17.11°, 17.91°, and 21.43° are characteristic peaks. The diffraction peaks at 2theta values of 15.33°, 19.29° and 20.81° are important peaks. The diffraction peaks at 2theta values of 12.82°, 27.27° and 25.47° are the next most important peaks.
[0091] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com