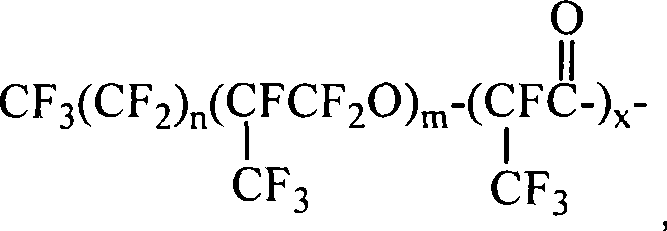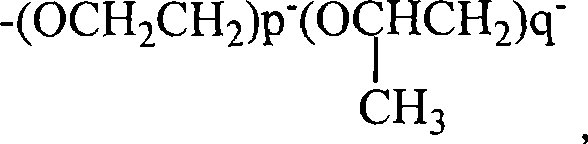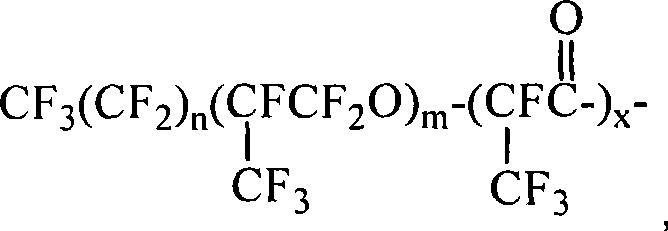Fluorine-containing block polymer and synthesis thereof
A technology of block copolymers and synthesis methods, applied in chemical instruments and methods, chemical/physical processes, transportation and packaging, etc., can solve problems such as endangering human life and health, restricting application expansion, low solubility, etc., and achieve low critical glue Beam concentration, high product purity, and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 300ml of diethylene glycol dimethyl ether, 60ml of tetramethylethylenediamine, 2g of potassium fluoride, and 2ml of triethylamine were introduced into a 2L stainless steel autoclave, and the reaction temperature was set at 20°C. Time 1h. Continue to stir for 30 minutes after the reaction is over, let stand for 30 minutes, and discharge. Stand for stratification, separate liquids, take the lower layer liquid, analyze the composition by gas chromatography, rectify, collect products of different fractions, and obtain oxaperfluoroalkyl fluorides with different chain lengths.
[0029] Take by weighing 20g (0.01mol) dry polyethylene glycol 2000, join in the 100ml flask that has tetrafluoro mechanical stirring device, constant pressure dropping funnel, thermometer, nitrogen protection, with 25ml anhydrous tetrahydrofuran as esterification solvent, Add 2g (0.02mol) of anhydrous triethylamine to absorb the generated hydrogen fluoride, start mechanical stirring for 30min, heat t...
Embodiment 2
[0032] The intermediate product oxaperfluoroalkyl fluoride: perfluoro-2,5-dimethyl-3,6-dioxononanoyl fluoride was prepared by the above method.
[0033]Take by weighing 16g (0.04mol) dry polyethylene glycol 400, join in the 100ml flask that has tetrafluoro mechanical stirring device, constant pressure dropping funnel, thermometer, nitrogen protection, with 20ml anhydrous tetrahydrofuran as esterification solvent, Add 8.1g (0.08mol) of anhydrous triethylamine to absorb the generated hydrogen fluoride, start mechanical stirring for 30min, heat in a constant temperature water bath to 50°C, and slowly drop in 39.85g (0.08mol) of perfluoro-2 with a constant pressure dropping funnel, 5-Dimethyl-3,6-dioxononanoyl fluoride was added dropwise within 3 hours, and the reaction was continued at 50°C for 6 hours. After the reaction was completed, filter three times with basic alumina, and distill off the solvent to obtain a yellow liquid water-soluble triblock fluorocarbon surfactant:
[...
Embodiment 3
[0036] Acid chlorides containing A blocks where n=2, m=5, x=1 are commercially available. The product containing the B segment with one end group being an amine group was purchased, the molecular weight was 1000, and p / q=2.
[0037] Weigh 100g (0.1mol) of the dry product containing the B segment, add it to a 500ml flask equipped with a tetrafluoro mechanical stirring device, a constant pressure dropping funnel, a thermometer, and nitrogen protection, and use 400ml of anhydrous nitrogen methyl pyrrolidone as the ester To dissolve the solvent, add 10.1g (0.1mol) of anhydrous triethylamine to absorb the generated hydrogen chloride, start mechanical stirring for 30mins, heat the constant temperature oil bath to 100°C, and slowly drop in 116.2g (0.1mol) of the above-mentioned The acid chloride containing block A was added dropwise within 2 hours, and the reaction was continued at 100°C for 10 hours. After the reaction is completed, filter with basic alumina three times, and distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com