Cationic polymer gene carrier and preparation method and application thereof
A cationic polymer, gene carrier technology, applied in the field of medicine, can solve the problems of non-targeting, high production cost of viral vectors, and immunogenicity, etc. Effect of Transfection Efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
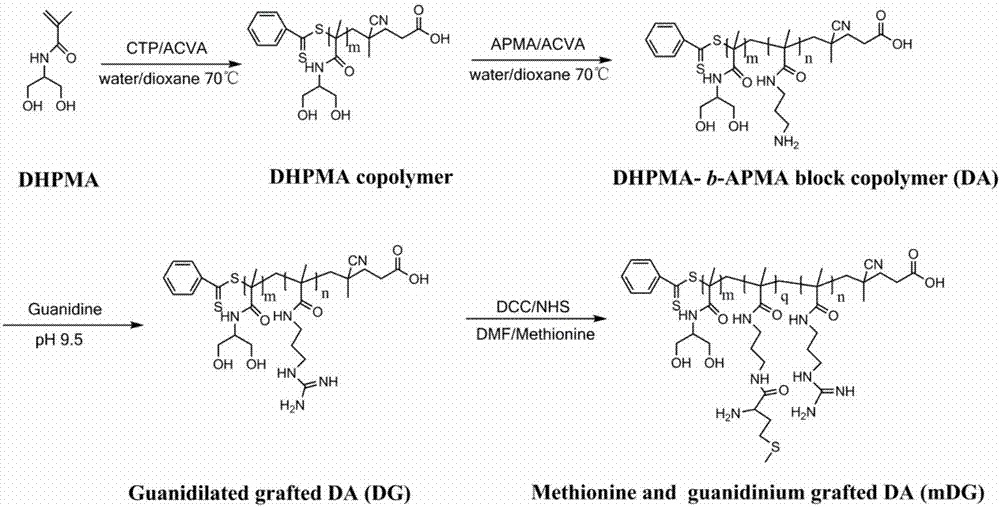
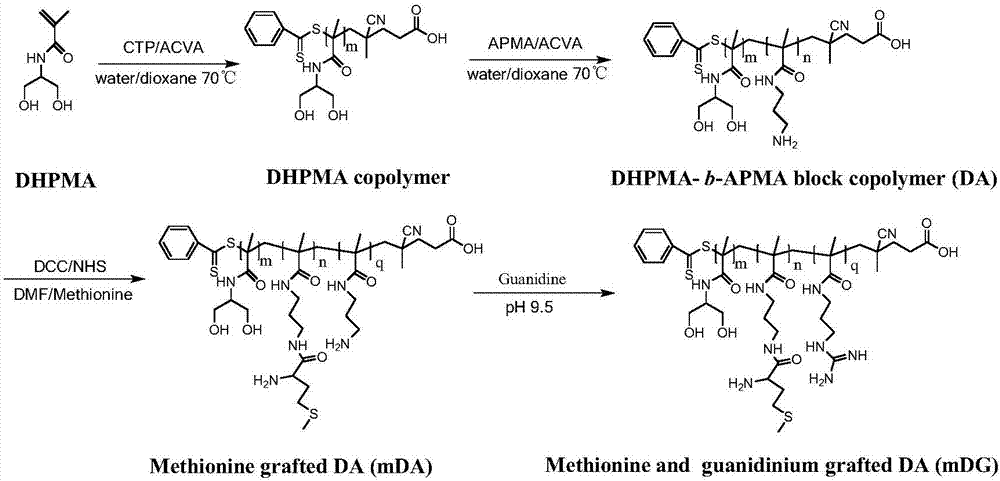
[0079] Cationic polymer carrier of embodiment 1 gene medicine and its preparation
[0080] (1) Preparation of poly DHPMA polymer:
[0081] DHPMA (1.0430g, 7.0mmol) was dissolved in 1.0mLmL distilled water, and then added to a 5mLmL polymerization vial, dithiobenzoic acid (4-cyanovaleric acid) ester (0.0336g, 0.12mmol), ACVA (0.0167g, 0.06 mmol) was dissolved in 0.5 mL of 1,4-dioxane and added to the polymerization vial. The system was ventilated with dry nitrogen for 1 h, and the polymerization vial was sealed with wax. Put the polymerization bottle into an oil bath preheated to 70°C for 24 hours. The polymerization tube was taken out and cooled in a cold pump at 0°C to terminate the polymerization reaction and obtain the product. The product was added to ice acetone to precipitate, centrifuged, and dissolved / precipitated repeatedly three times, and the product was dried in a vacuum oven at 50° C. to obtain 0.0362 g (yield: 72%) of the product poly-DHPMA polymer (PDHPMA). ...
Embodiment 2
[0092] The cationic polymer carrier of embodiment 2 gene medicine and its preparation
[0093] (1) Preparation of poly DHPMA polymer:
[0094] With the first (1) step of embodiment one
[0095] (2) Preparation of polyDA polymer:
[0096] With the first (2) step of embodiment one
[0097] (3) Synthesis of guanidinated DG polymer:
[0098] Take DA polymer (1.78g, 0.1mmol) and dissolve in water, add guanidinating reagent 1-H-pyrazole-1-carboxamidine hydrochloride (0.059g, 0.4mmol), and adjust the pH of the reaction solution with saturated sodium carbonate solution To 9.0, react at room temperature for 24h. The reaction solution was added to an ultrafiltration tube (10 kd), washed with ultrapure water three times, and 1.23 g of DG polymer was freeze-dried (yield: 67%).
[0099] Table 3 shows the polymer compositions and average molecular weights of polymers DG1, DG2, and DG3 prepared according to the above method with different segment ratios.
[0100] Table 3 Composition an...
Embodiment 3
[0105] Example 3 Preparation and Characterization of mDG / siRNA Complex
[0106] (1) Preparation of mDG / siRNA complex:
[0107] Dissolve quantitative siRNA stock solution in enzyme-free water, prepare mDG / siRNA complex according to the molar ratio of amino nitrogen in mDG polymer to the molar ratio of phosphate in siRNA (siRNA1, siRNA2, siRNA3 and siRNA-NC), and take Add siRNA (any one of siRNA-1, siRNA-2, siRNA-3, siRNA-NC) stock at the N / P ratio of 1:1, 1:4, and 1:8 to mDG1, mDG2, and mDG3 polymers The solution was mixed and incubated at room temperature for 30 min to prepare a series of DHPMA-b-GPMA / siRNA complexes (i.e. complexes of any two of mDG1, mDG2, mDG3 and siRNA-1, siRNA-2, siRNA-3, siRNA-NC), and then The complex solution was sonicated and passed through a 0.22 μm filter membrane and stored at 4°C for later use.
[0108] (2) Agarose gel electrophoresis of mDG / siRNA complex
[0109] Mix mDG polymer and siRNA according to the charge ratio of 1:4, 1:8 and 1:16, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com