A kind of colorimetric detection method of primaquine drugs
A technology of primaquine and detection method, applied in the field of medicine, can solve the problems of interfering with PMQ determination and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Grimess reaction of primaquine
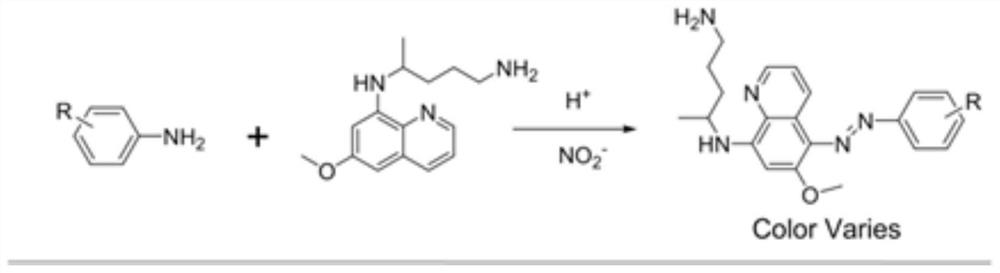
[0035]Dissolve the aniline and 1-fold primaquine (PMQ) in 5% phosphoric acid, followed by the slow addition of sodium nitrite at room temperature (25 °C). The formation of colored azo products can be easily monitored by naked eye observation or TLC (e.g., t.g., TLC Figure 1 )。
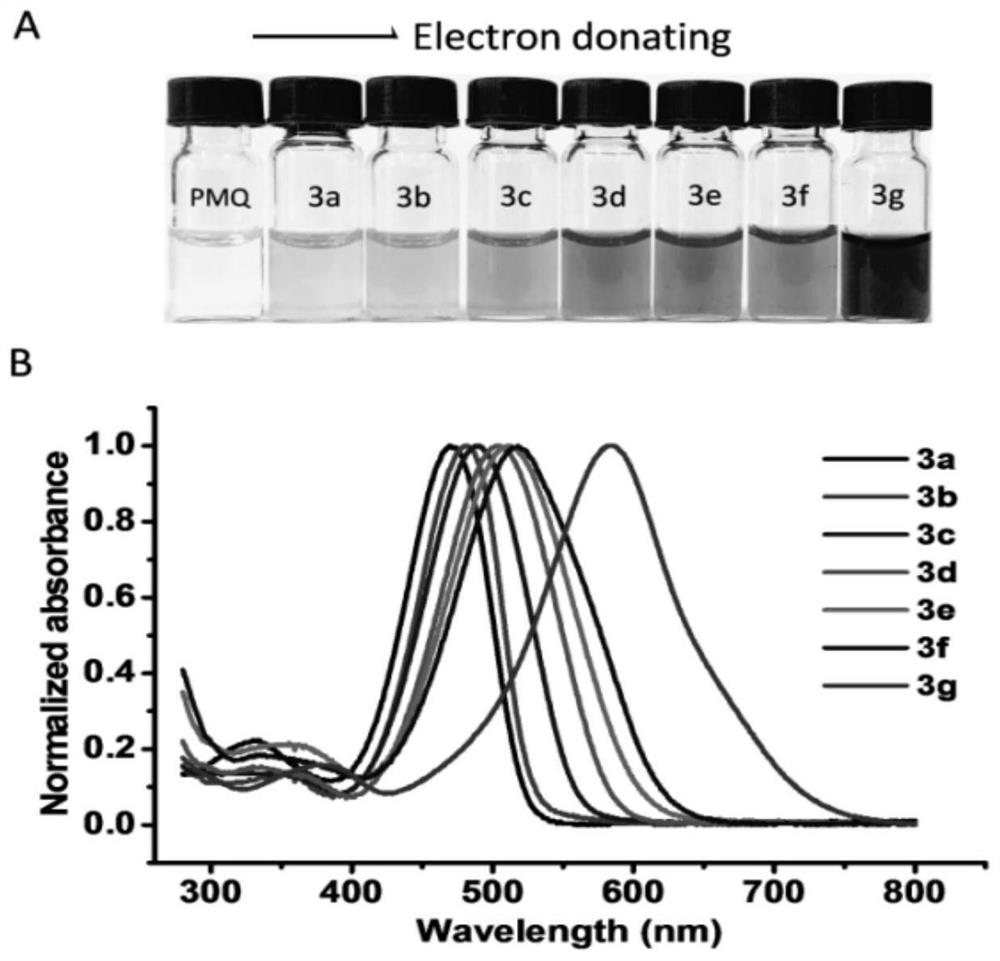
[0036] The reaction is carried out using aniline with various substituents in the benzene ring. Changing the substituent group can form products of different colors. as Figure 2 As shown, substitutions with electron-giving effects can essentially cause redshifts in the UV-Vis absorption spectrum. The spectrum of the strongly powered methoxy-substituted reaction product 3d has the strongest absorption at 504 nm, while the spectrum of the product 3a of strongly absorbed electron sulfonamide has the strongest absorption at 470 nm. The power supply of methyl groups is lower than that of methoxy, but it is still much stronger than sulfonamides, nitro groups and ...
Embodiment 2
[0042] Example 2 tests the change in absorbance and time of the colored product
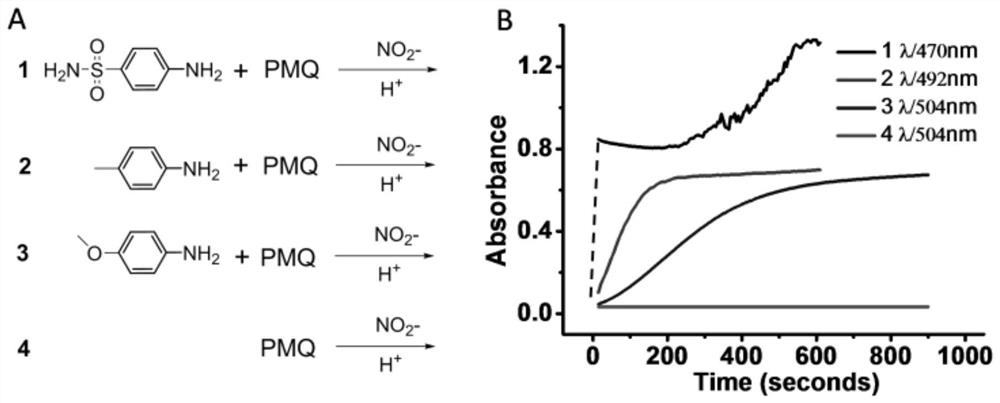
[0043] PMQ can react with various anilines to form colored products, and then use this color development reaction to determine PMQ colorimetric. Tested with aniline with different electrical substituents (R) (sulfonyl 2a, methyl 2c and methoxy 2d). To test absorbance versus change over time, first mix 100 μL of aniline solution (200 mM dissolved in 0.2 M HCl) with 50 μL of PMQ (50 μM) and then add 50 μL of aqueous sodium nitrite (5 mM). The dynamic change in absorbance of each aniline at maximum wavelength is recorded by a multifunctional microplate reader.
[0044] as Figure 3 As indicated, the reaction in 2a is faster than the other two anilines. The reaction rate of 2c is higher than 2d but below 2a, mainly because the electron-giving effect of methyl is somewhere in between. As mentioned earlier, the same trend was observed on the displacement of the UV-Vis absorption peak. What's more, PMQ itsel...
Embodiment 3
[0046] Example 3Griess reaction to selectivity of PMQ
[0047] The antimalarial drugs 4-aminoquinoline mefloquine (MQ), chloroquine (CQ), piperaquine (PIP) and dihydroartemisinin (DHA) were used to participate in the Griess reaction alone, or other antimalarial drugs were mixed with PMQ to participate in the reaction. The results are as follows Figure 5 as shown, absorbance value I 504 There was no response to MQ, CQ, PIP, and DHA (all 50 μM), but to drug mixtures containing PMQ (50 μM). The results showed that the Grices-based response method was highly selective for PMQ and could distinguish between other antimalarial drugs commonly used in malaria combinations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com