A kind of oral pharmaceutical composition for treating atherosclerosis
A technology of atherosclerosis and composition, applied in the field of medicine, can solve problems such as inconvenient drug taking, side effects, and complex ingredients of traditional Chinese medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
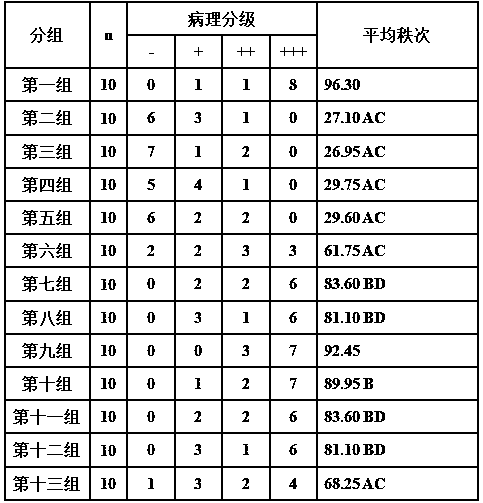
[0036] Example 1 Granules for treating atherosclerosis and its preparation
[0037]
[0038] Preparation:
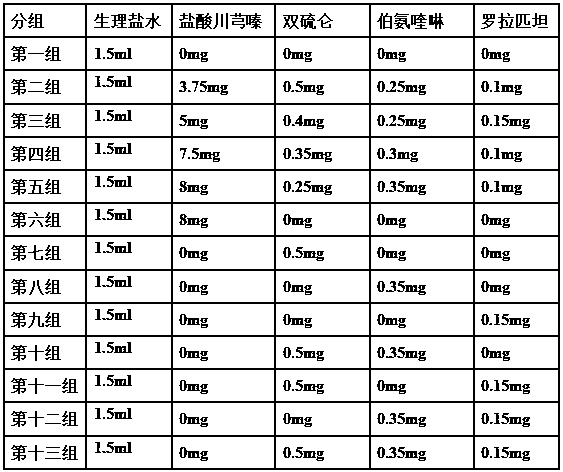
[0039] A: Take ligustrazine hydrochloride, disulfiram, primaquine, and rorapitant and pass through a 100-mesh sieve, and mix well.
[0040] B: Take lactose, microcrystalline cellulose, and crospovidone to pass through a 100-mesh sieve, and mix well. which milk
[0041] The dosage of sugar is 2.0 times of the weight of ligustrazine hydrochloride; the dosage of microcrystalline cellulose is 1.2 times of the weight of ligustrazine hydrochloride; the dosage of crospovidone is 0.12 times of the weight of ligustrazine hydrochloride.
[0042] C: Mix the powder obtained in step A and step B evenly.
[0043] D: Take povidone K30 and add water and stir well to make a liquid dispersion system. Wherein the consumption of povidone K30 is 0.17 times of the weight of ligustrazine hydrochloride; the consumption of water is 32.3 times of the weight of povidone K30.
[0044] E: Th...
Embodiment 2
[0046] Example 2 Granules for treating atherosclerosis and its preparation
[0047]
[0048] Preparation:
[0049] A: Take ligustrazine hydrochloride, disulfiram, primaquine, and rorapitant and pass through a 100-mesh sieve, and mix well.
[0050] B: Take lactose, microcrystalline cellulose, and crospovidone to pass through a 100-mesh sieve, and mix well. where lactose is used
[0051] The amount is 2.0 times of the weight of ligustrazine hydrochloride; the amount of microcrystalline cellulose is 1.2 times of the weight of ligustrazine hydrochloride; the amount of crospovidone is 0.12 times of the weight of ligustrazine hydrochloride.
[0052] C: Mix the powder obtained in step A and step B evenly.
[0053] D: Take povidone K30 and add water and stir well to make a liquid dispersion system. Wherein the consumption of povidone K30 is 0.17 times of the weight of ligustrazine hydrochloride; the consumption of water is 32.3 times of the weight of povidone K30.
[0054] E: ...
Embodiment 3
[0056] Example 3 Granules for treating atherosclerosis and its preparation
[0057]
[0058] Preparation:
[0059] A: Take ligustrazine hydrochloride, disulfiram, primaquine, and rorapitant and pass through a 100-mesh sieve, and mix well.
[0060] B: Take lactose, microcrystalline cellulose, and crospovidone to pass through a 100-mesh sieve, and mix well. which milk
[0061] The dosage of sugar is 2.0 times of the weight of ligustrazine hydrochloride; the dosage of microcrystalline cellulose is 1.2 times of the weight of ligustrazine hydrochloride; the dosage of crospovidone is 0.12 times of the weight of ligustrazine hydrochloride.
[0062] C: Mix the powder obtained in step A and step B evenly.
[0063] D: Take povidone K30 and add water and stir well to make a liquid dispersion system. Wherein the consumption of povidone K30 is 0.17 times of the weight of ligustrazine hydrochloride; the consumption of water is 32.3 times of the weight of povidone K30.
[0064] E: Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com