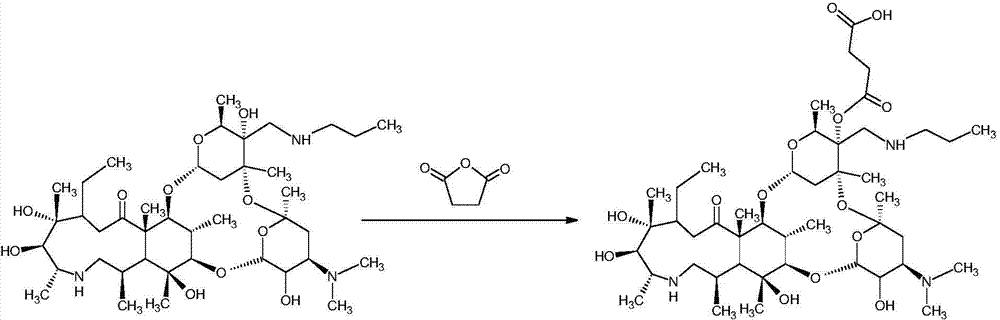
Synthesis method of half-antigen and complete antigen of tulathromycin
A telamycin and complete antigen technology, applied in the field of immunology, can solve the problems of no telamycin enzyme-linked immunosorbent assay method, etc., and achieve the effect of high titer, simple preparation method and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1 telamycin hapten
[0026] Use the succinic anhydride method to derivatize telamycin, and the specific steps are as follows: take 4 mg telamycin standard and dissolve it in 500 μl anhydrous pyridine, add 1 mg succinic anhydride, reflux and stir for 6 hours at 114 ° C, and dry the reaction solution with nitrogen , That is, the hapten of Tyramycin.
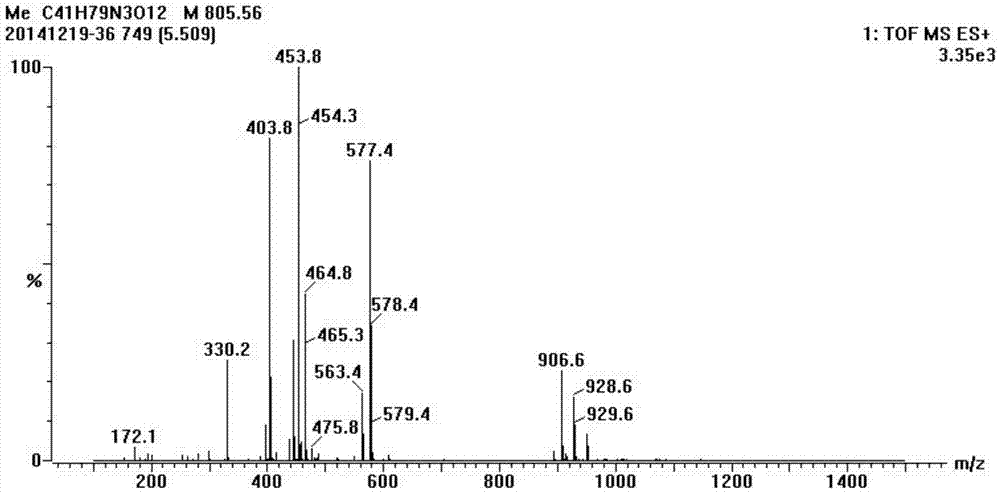
[0027] The obtained telamycin hapten was identified by LC-MS. The mass spectrum of the telamycin hapten is shown in figure 2 shown. The molecular weight of telamycin is 806.08, and the molecular weight of succinic anhydride is 100.07. According to theoretical speculation, the molecular weight of the derivative obtained after the reaction of the hydroxyl group in the telamycin structure with succinic anhydride is 906.08. From figure 2 It can be seen that there is a peak with a mass-to-charge ratio of 906.6 in the derivative, which is consistent with the peak obtained in theory and practice, ...
Embodiment 2
[0028] Synthesis of embodiment 2 telamycin hapten
[0029] Dissolve 5 mg of standard telamycin in 400 μL of anhydrous pyridine, add 2 mg of succinic anhydride, reflux and stir at 90-114°C for 9 hours, and dry the reaction solution to obtain the telamycin hapten.
Embodiment 3
[0030] The synthesis of the complete antigen of embodiment 3 Tyramycin
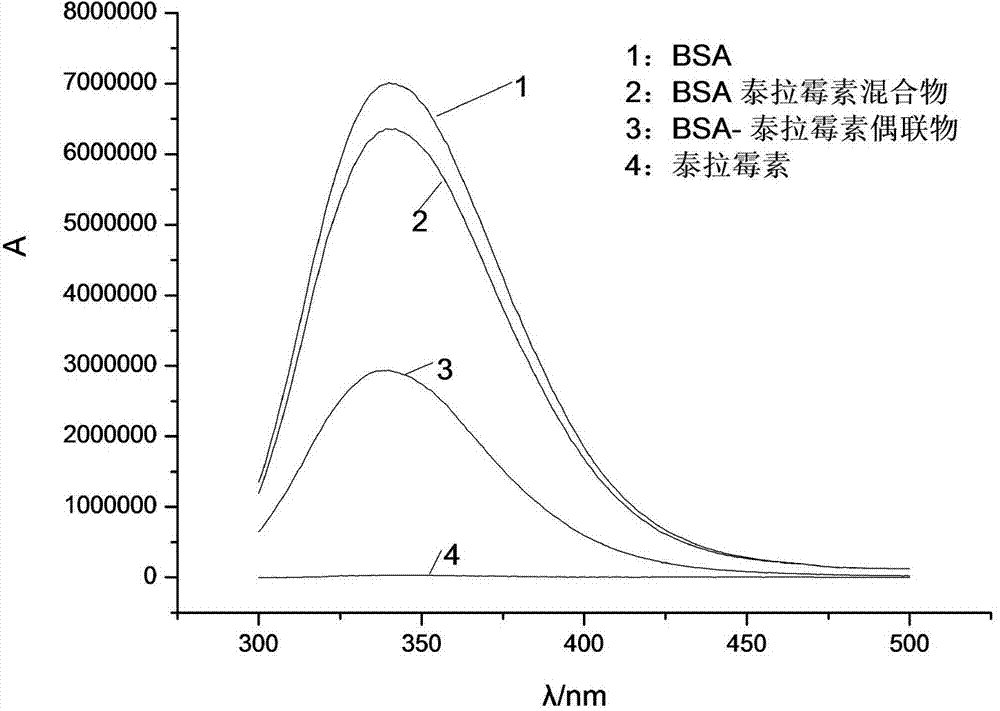
[0031] The complete antigen was synthesized by the active ester method. 1 mg of the product derived from telamycin (ie, hapten), 0.6 mg of EDC, and 0.4 mg of NHS were dissolved in 300 μl of DMF, and stirred at room temperature for 30 min in the dark. 6 mg of BSA was dissolved in 2 mL of PBS, and the activated telamycin derivative was added dropwise to the BSA solution while stirring, and reacted overnight at 4°C. After the reaction, dialyze with 0.01mol / L PBS for 3 days and change the solution every 6 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com