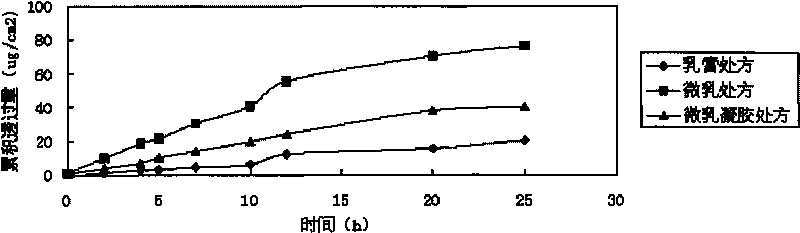
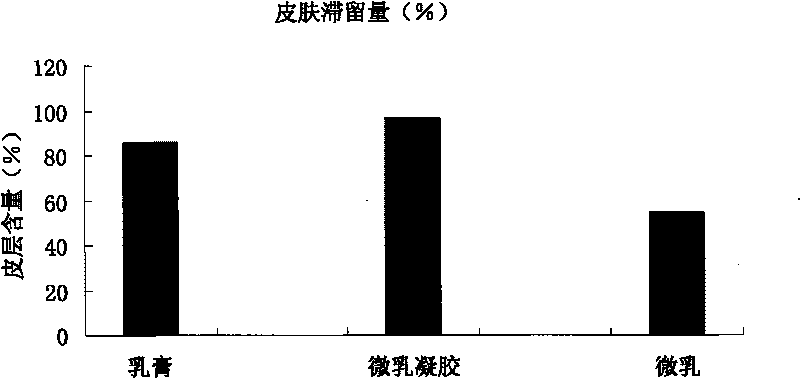
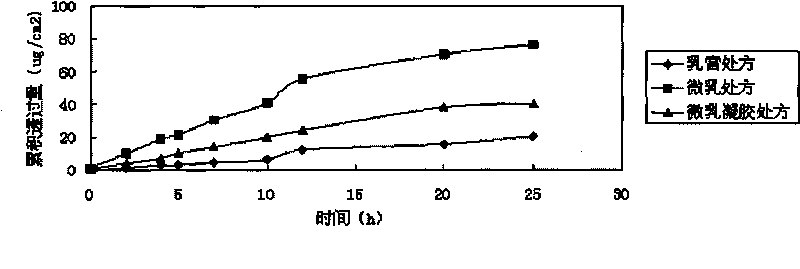
Imiquimod micro emulsion gels for local skin and preparation method thereof
A technology of imiquimod micro, emulsion gel, applied in skin diseases, medical preparations containing active ingredients, antiviral agents, etc., can solve side effects, high transdermal absorption, and low local skin drug storage and other problems, to achieve the effect of enhancing compliance, good compatibility, and conducive to percutaneous penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 100g of imiquimod, 1.5kg of oleic acid and stir to dissolve, then add 3.0kg of castor oil polyoxyethylene ether EL60 and 1kg of isopropanol, mix and stir evenly, add about 60kg of water to the container containing the above solution under constant stirring Gently stir in the homogenizer to form a light yellow to bluish transparent oil-in-water microemulsion A with an average particle size of 30nm; another 1.5kg of Carbomer 934 powder is weighed and evenly sprinkled in 50kg of distilled water. Fully swell, prepare carbomer water swelling solution B containing 3% carbomer 934 for later use; weigh 33.4kg of B and add it to A, stir while adding, add about 1kg of triethanolamine to adjust the pH value, and obtain 100kg of transparent Imiquimod microemulsion gel, that is, 0.1% imiquimod microemulsion gel.
Embodiment 2
[0025] Take 100g of imiquimod, 1.5kg of oleic acid and stir to dissolve, then add 1.5kg of castor oil polyoxyethylene ether EL60 and 1kg of isopropanol, mix and stir evenly, add about 10kg of water to the container containing the above solution under constant stirring Gently stir in the homogenizer to form a light yellow to bluish transparent bicontinuous microemulsion A with an average particle size of 30nm; another 300g of Carbomer 934 powder is weighed, evenly sprinkled in 10kg of distilled water, fully Swell, prepare carbomer water swelling solution B containing 3% carbomer 934 for later use; weigh 5.78g of B and add it to A, stir while adding, add about 120g of triethanolamine to adjust the pH value, and obtain 20kg of transparent rice Quinomod microemulsion gel, that is, 0.5% imiquimod microemulsion gel.
Embodiment 3
[0027] Take 100g of imiquimod, 1.5g of oleic acid and stir to dissolve, then add 1.5kg of castor oil polyoxyethylene ether EL60 and 1.0kg of isopropanol, mix and stir evenly, add about 3.0kg of water to the The solution was slightly stirred in a homogenizer to form a light yellow transparent water-in-oil microemulsion A with an average particle size of 30nm; another 300g of Carbomer 934 powder was weighed, evenly sprinkled in 10kg of distilled water, fully swelled, and prepared Prepare carbomer water swelling solution B containing 3% carbomer 934 for later use; weigh 2.84kg of B and add it to A, stir while adding, add about 60g of triethanolamine to adjust the pH value, and prepare 10kg milky white imiquimod Microemulsion gel, namely 1% imiquimod microemulsion gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com