Preparation method of imiquimod
A technology of hydroxyquinoline and isobutylaminoquinoline, applied in the direction of organic chemistry, etc., can solve the problem of 3-amino-4-isobutylaminoquinoline quality decline, large safety hazard of fuming nitric acid, and reduced product yield and quality issues, to achieve the effect of no waste liquid discharge production, reduce one-step operation, and increase yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
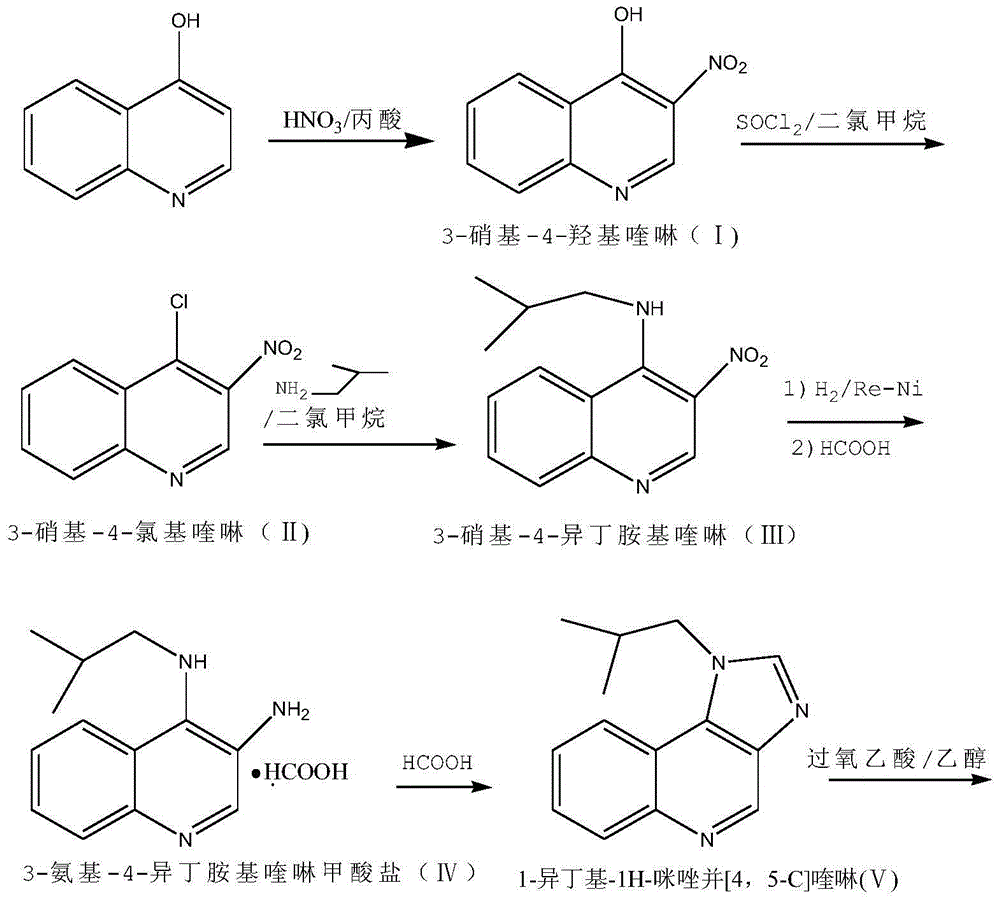
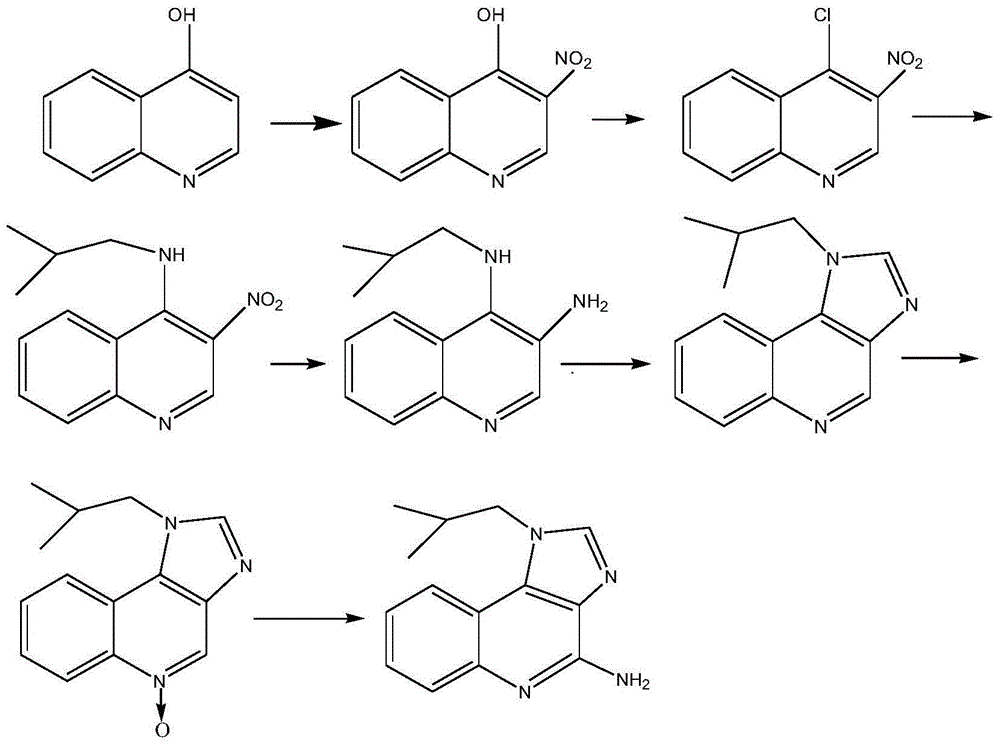
[0045] Preparation of 3-Nitro 4-Hydroxyquinoline (Ⅰ)
[0046] Stir and add 10kg of 4-hydroxyquinoline to 100kg of propionic acid, heat up to about 50°C while stirring, the solid dissolves, continue to slowly heat up to 105°C, start to drop 9kg of nitric acid with a mass concentration of 68%, and keep the reaction during the dropwise addition The temperature in the tank was about 105°C, and the dropwise addition was completed in about 1.5 hours.
[0047] After dripping, reflux the reaction solution for another 30 minutes, lower the temperature to about 20°C, and filter with suction to obtain about 89 kg of mother liquor, which will be kept for use. At the same time, a solid filter cake will be obtained. Wash the filter cake with a large amount of water until the pH value is 5-6, and filter to dry Finally, the filter cake was dried at about 90°C to obtain 12.1 kg of a light yellow solid, namely 3-nitro4-hydroxyquinoline (I), with a yield of 92.4%.
[0048] m.p>300℃ Literature m...
Embodiment 2
[0051] Preparation of 3-Nitro-4-Hydroxyquinoline (Ⅰ) by Circulating Mother Liquor
[0052] The 89kg mother liquor obtained by the above-mentioned suction filtration is sucked in the distillation tank (model), and the temperature is raised to carry out normal pressure distillation. When the distillation temperature rises to 140° C., the distillation is stopped to obtain 15.7 kg of the distillation product. After cooling down slightly, 27 kg of propionic acid is added, thereby dissolving the mother liquor to recycle.
[0053] Then use the recovered mother liquor to carry out the next round of reaction. Below 100 DEG C, add 10kg 4-hydroxyquinoline again in reclaiming mother liquor, while stirring, be warmed up to 105 DEG C, begin to drip the nitric acid 9kg that mass concentration is 68%, keep about 115 DEG C in the dripping process, about 1.5 The dropwise addition is completed within 1 hour, and then reflux reaction for 30 minutes after the dripping is completed, the temperatur...
Embodiment 3
[0058] Preparation of 3-nitro-4-isobutylaminoquinoline (Ⅲ)
[0059] Add 100kg of dichloromethane and 5kg of dimethylformamide into a dry and clean reaction tank in turn, start stirring and add 10kg of nitrates, 8.5kg of thionyl chloride, start heating, reflux reaction for 3 hours, TLC detection (thin film chromatography ), when no 3-nitro 4-hydroxyquinoline was detected, the reaction was complete.
[0060] Stop heating, cool down with ice-salt water, keep the temperature below 0°C, and add triethylamine dropwise to adjust the pH value to 7-8, continue to keep the temperature below -5°C, and directly add 4.5kg of isobutylamine and 7.3kg of triethylamine dropwise The mixed solution of amine was added dropwise in 2 hours.
[0061] After dripping and naturally warming up to room temperature, heat and reflux for 30 minutes, add 50kg of water, stir for 10 minutes, leave to separate layers, keep the water layer for use, and wash the organic layer three times with 50kg of 0.5% sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com