Crystal, preparation method and applications of crystal
A crystal form and recrystallization technology, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, metabolic diseases, etc., can solve problems such as interference, and achieve the effect of reducing diabetic complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
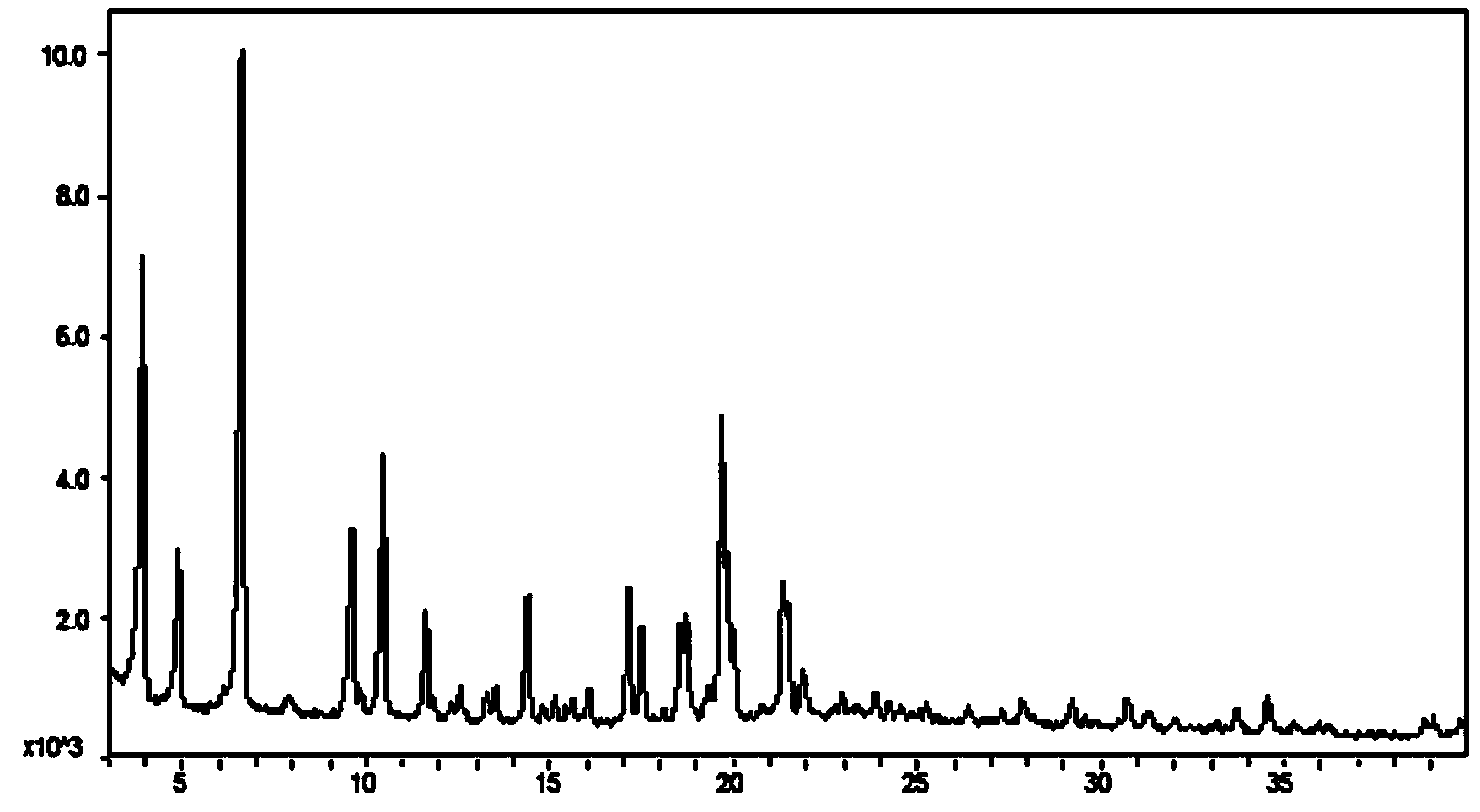
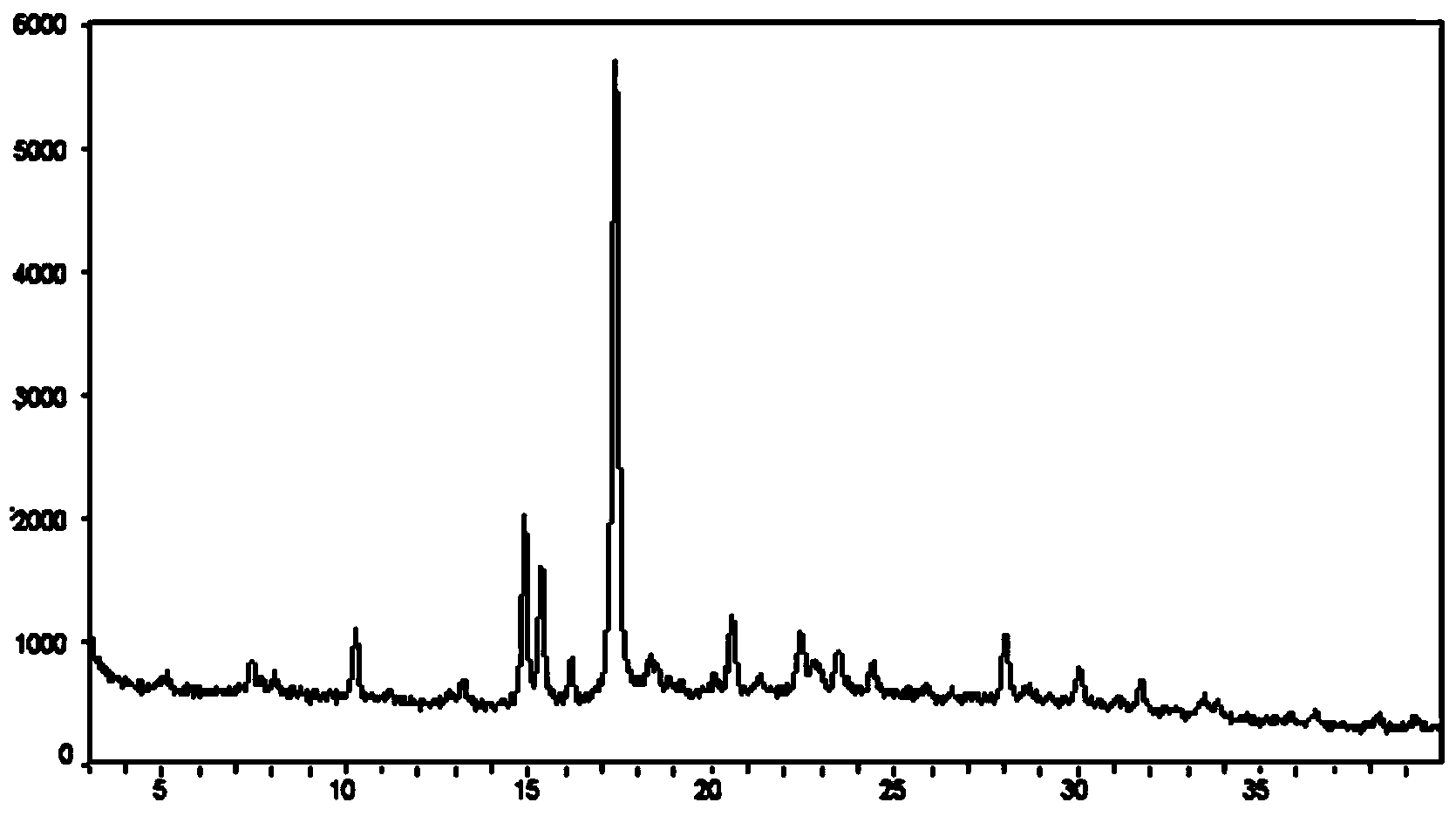
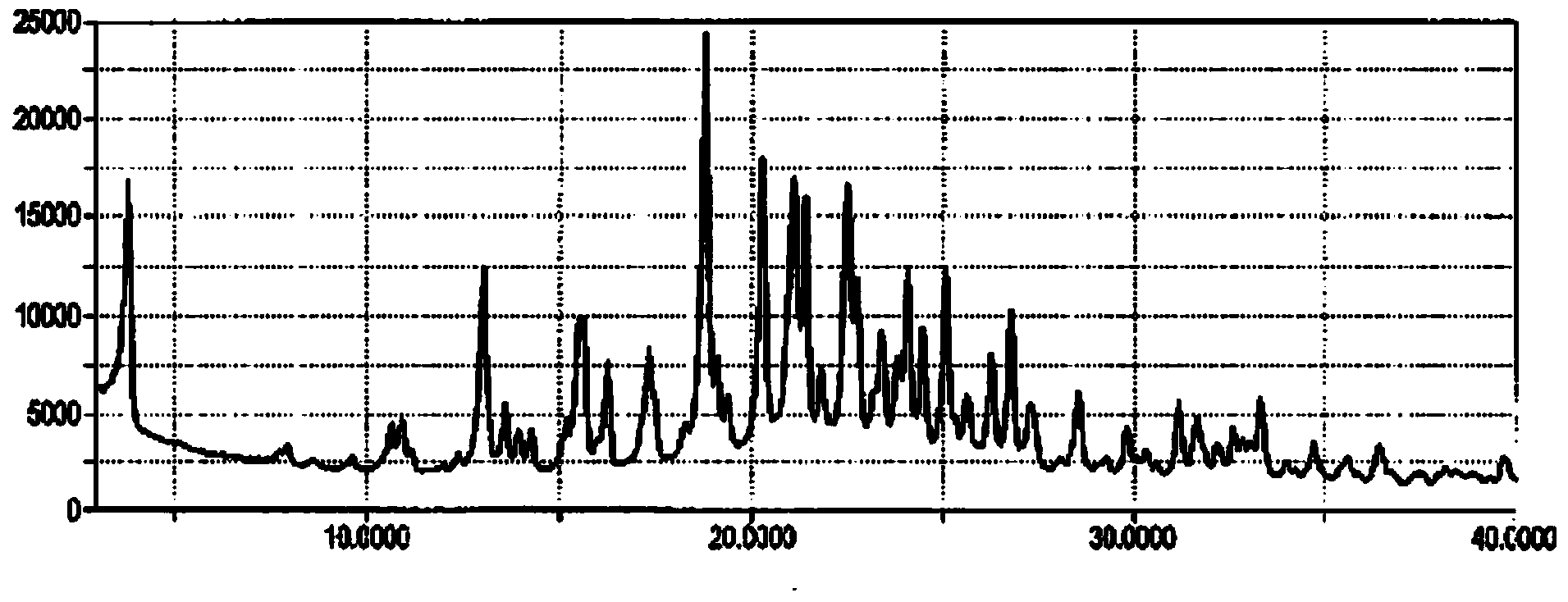
[0037] Weigh about 10mg of 1-(b-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene into a glass bottle, at room temperature Add 0.4mL of n-octanol to dissolve the sample until clear, then place the sample at 5°C overnight (12h), the solid precipitates out, put the suspension in a filter centrifuge tube, and centrifuge at 4000rpm for 3 minutes to obtain The solid was detected by XRPD according to the method specified in this patent, and octanol and 1-(b-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)- The molar ratio of 2-thienylmethyl]benzene. The XRPD results show that the obtained solid position is crystal form III, and octanol and 1-(b-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl Base] The molar ratio of benzene is 0.5:1. X-ray powder diffraction in this patent, the operation and analysis steps are as follows:
[0038] The X-ray powder diffraction pattern was collected on a Bruker D8 Discover X-ray powder diffractometer of GADDS...
Embodiment 2
[0044] Weigh about 10mg of 1-(b-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene into a glass bottle, at room temperature Add 0.5mL of n-octanol to dissolve the sample until clear, then gradually add 3.05mL of n-heptane, crystallize and precipitate a solid, place the suspension in a filter centrifuge tube, and centrifuge at 4000rpm for 3 minutes to obtain a solid. The XRPD method is used for detection, and it is crystal form III.
Embodiment 3
[0046] Weigh about 10mg of 1-(b-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene into a glass bottle, at room temperature Add 0.5 mL of n-octanol to dissolve the sample until clear, take 3 mL of anti-solvent into a 20 mL glass bottle, put the 1.5 mL glass bottle containing the n-octanol solution of the compound into a 20 mL glass bottle, and let the anti-solvent gas diffuse Put the suspension into the sample solution, place the suspension in a filter centrifuge tube, and centrifuge at 4000rpm for 3 minutes to obtain a solid that is detected by the XRPD method described in Example 1 and is crystal form III. Here, n-hexane, pentane, cyclohexane, toluene, n-heptane, etc. can also be used as the anti-solvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com