Method for secretory production of glycoprotein having human-type sugar chain using plant cell
a technology of plant cells and glycoproteins, which is applied in the direction of peptide sources, transferases, enzymology, etc., can solve the problems of tobacco plant antibody protein decomposition, unstable, and antibody protein produced within the cell is decomposed by the protease,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0091] The present invention is described below by referring to the Examples. The following Examples are only to illustrate but not to limit the present invention.
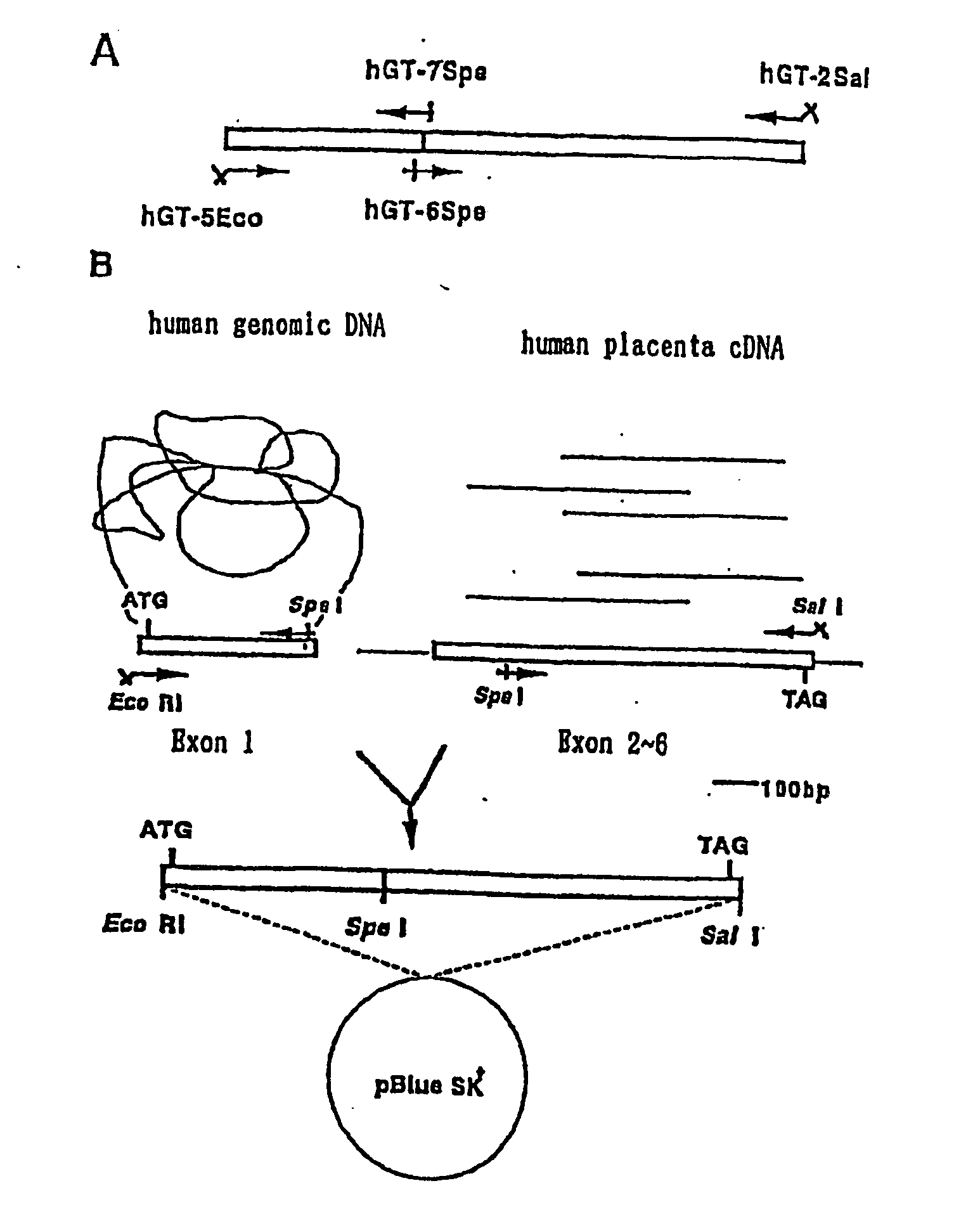
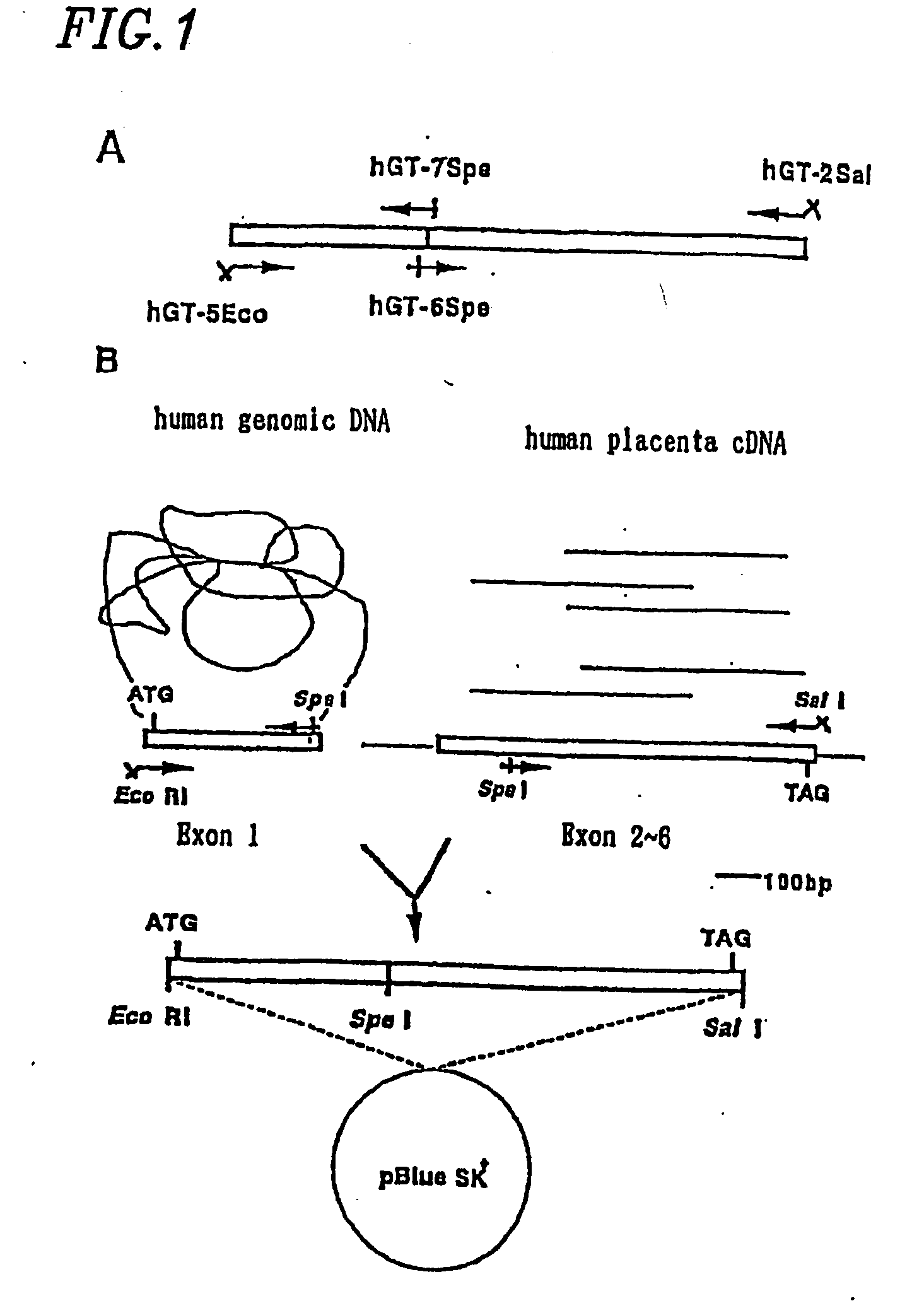
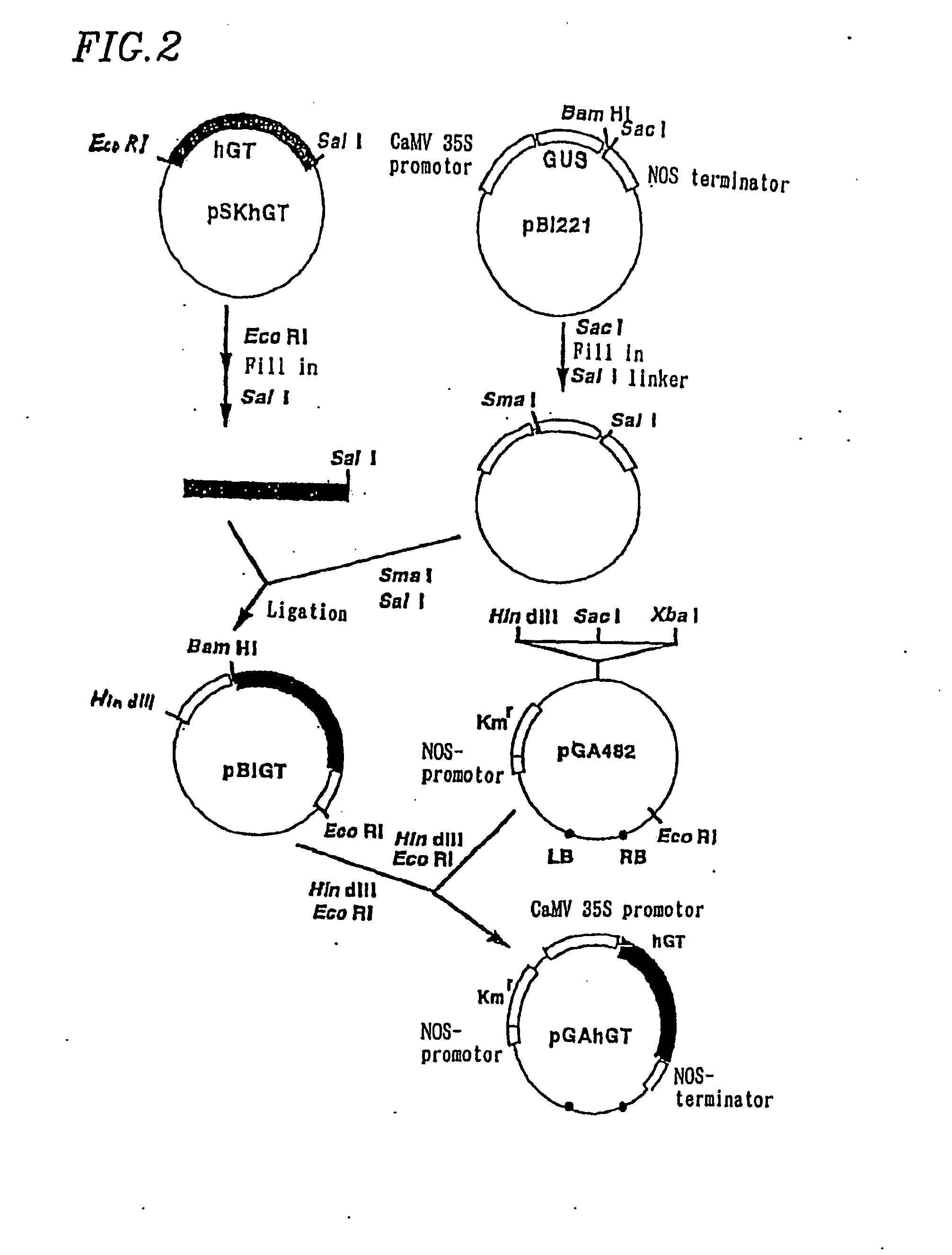
1. Cloning of Human β1-4 Galactose Transferase Gene
[0092] The β1-4 galactose transferase (hGt) (EC2.4.1.38) has been already cloned and a primary structure comprising 400 amino acids has been revealed (K. A. Masri et al., Biochem. Biophys. Res. Commun., 157, 657-663 (1988)).
(1) Primer Preparation and Template DNA
[0093] By referring to the report of Masri et al., the following primer were prepared.
(SEQ. ID NO: 1)hGT-5Eco:5′-AAAGAATTCGCGATGCCAGGCGCGCGTCCCT-3′(SEQ. ID. NO: 2)hGT-2Sal:3′-TCGATCGCAAAACCATGTGCAGCTGATG-5′(SEQ. ID. NO: 3)hGT-7Spe:3′-ACGGGACTCCTCAGGGGCGATGATCATAA-5′(SEQ. ID. NO: 4hGT6Spe:5′-AAGACTAGTGGGCCCCATGCTGATTGA-3′
[0094] As the template DNA, human genomic DNA, human placenta cDNA and human kidney cDNA purchased from Clontech were used.
[0095] Using two combinations of (i)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| fluorescence wavelength | aaaaa | aaaaa |
| fluorescence wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com