Human-type anti-blood coagulation factor VIII antibody
a technology human anti-fviii, which is applied in the field of can solve the problems of ineffective supplemental treatment of blood coagulation factor, difficult hemostatic management of hemophilia patients, and the inability to completely humanize the anti-fviii inhibitor antibody, and the antibody preparation of a complete human anti-fviii inhibitor antibody has not been successful
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Phage Library from Hemophilia Patient:
[0058]A phage library was constructed with reference to the method reported by J. D. Marks et al. (J. Mol. Biol., 222: 581–597, 1991).
[0059]Lymphocytes were separated with Ficoll from peripheral blood (28 ml) from a patient having the anti-FVIII inhibitor antibody, washed thoroughly with PBS, and treated with ISOGEN (Nippon Gene Co., Ltd.) to prepare a total RNA. The obtained total RNA was divided into three portions, each of which was reverse-transcribed with p primers specific for the constant regions of human IgG, K chain or λ chain with First Strand cDNA Synthesis Kit (Pharmacia bio tec) to prepare cDNAs for respective constant regions. The obtained cDNAs were then used as a template for amplifying antibody V region genes by polymerase chain reaction (PCR) using primers specific for gene families as a combination of VH+JH, VK+JK, and Vλ+Jλ, as reported by Marks et al.
[0060]VH genes and VK genes, and VH genes and Vλ genes, res...
example 2
Screening:
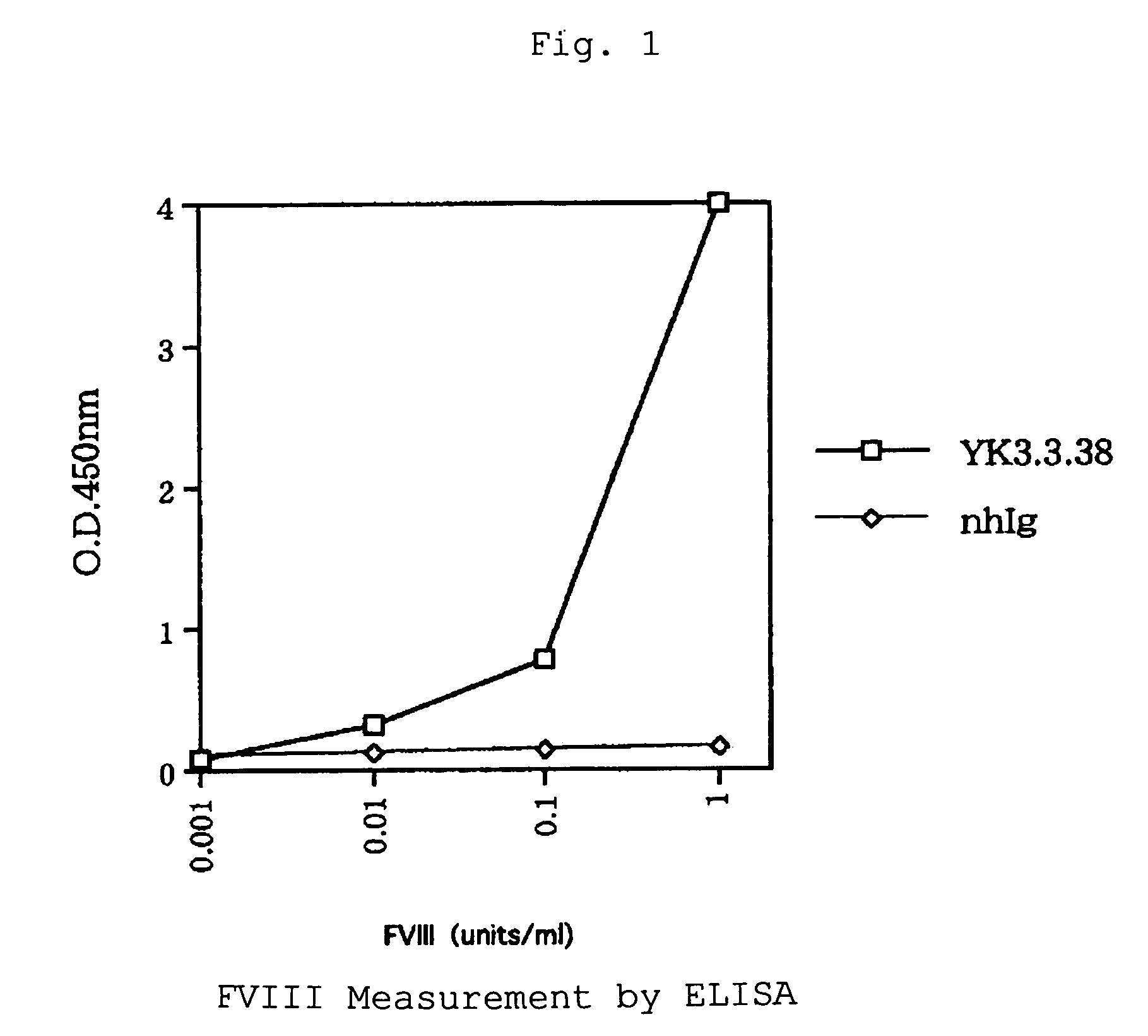
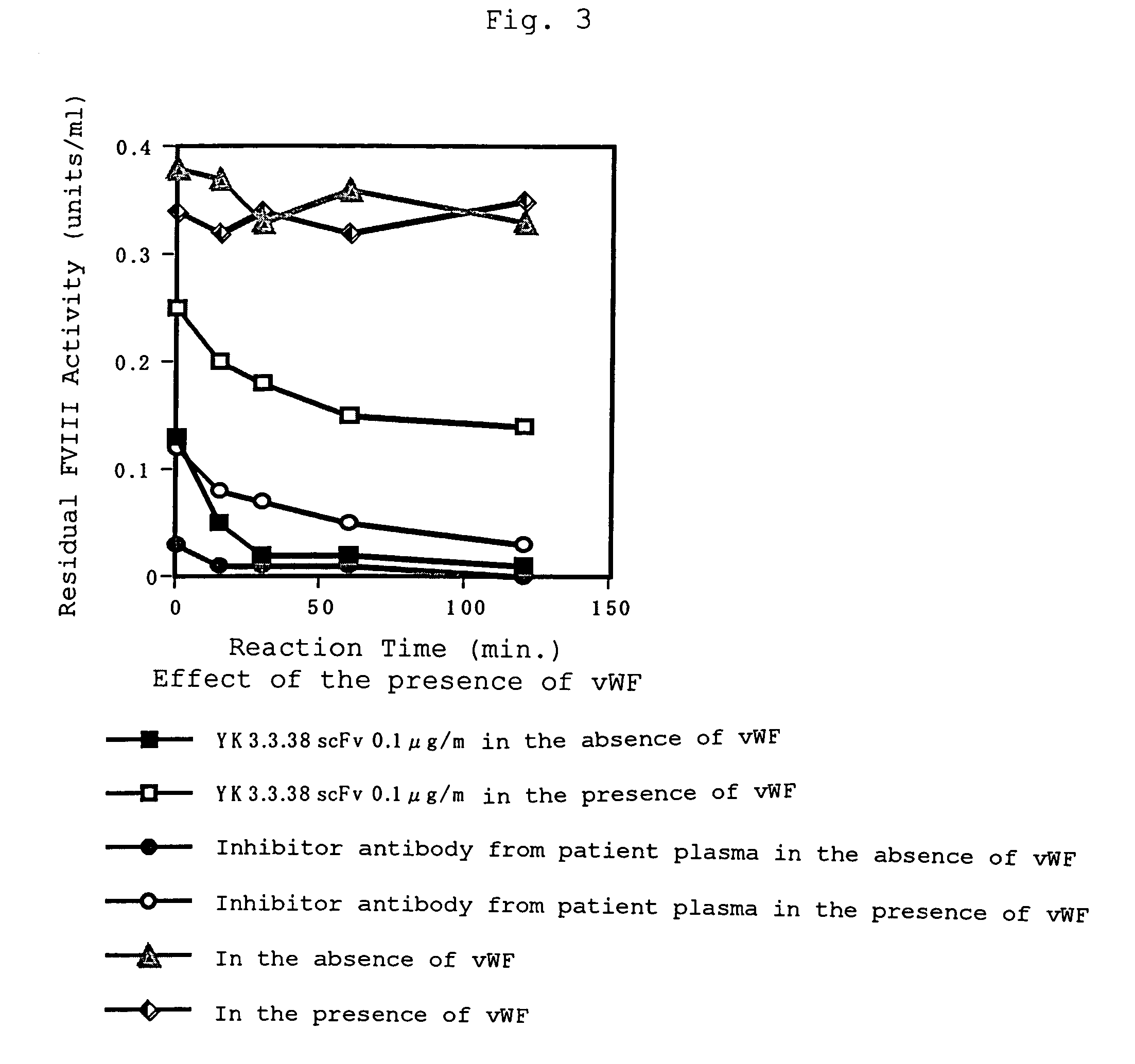
[0061]Factor VIII (Cross Eight M; 10 units / mL) dissolved in a screening buffer (4% skim milk, 0.5% bovine serum albumin, 50 μg / mL mouse IgG, 0.1% Tween 20, TBS; 500 μL) and either 0.5 μg / mL of anti-Factor VIII monoclonal antibodies conjugated with biotin FVIII:C14-182 (mouse; recognizing Factor VIII L chain) or FVIII:C14-282 (mouse; recognizing FVIII H chain A2 domain) dissolved in a screening buffer (500 μL) were mixed in a plastic tube (FALCON 2058) to react at room temperature for 30 minutes. Thereto was added the phage library displaying human antibody derived from patients (a solution of phage displaying single-chain antibodies; 1 mL) to react at room temperature for 1 hour and then at 4° C. overnight.
[0062]Avidin bound magnetic particles (Perseptive; 100 μL) treated with 2% skim milk / 0.05% Tween 20 / TBS were added to the above mixture of anti-FVIII monoclonal antibody conjugated with biotin / FVIII (1 mL) and the mixture was gently mixed upside-down for 15 minutes. The ...
example 3
Expression and Recovery of scFv:
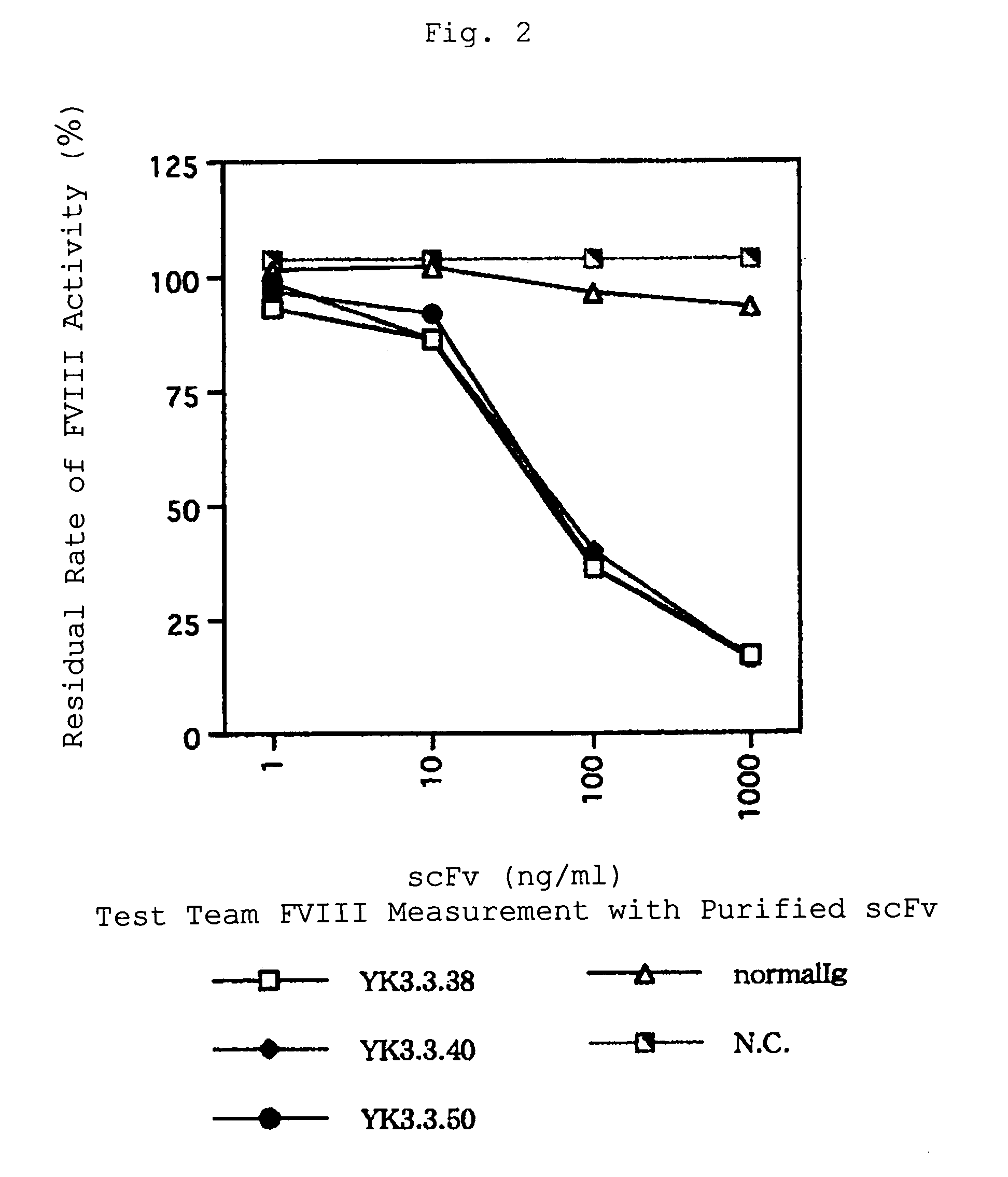
[0064]A soluble scFv was expressed with E. coli HB2151, recovered from E. coli periplasmic fraction and crudely purified. Affinity purification was performed with RAPAS Purification Module (Pharmacia Biotech) when further purification was needed.
[0065]Purity of the purified scFv protein was confirmed by SDS-polyacrylamide gel electrophoresis and Western blotting targeted to E-Tag epitope at the C-terminal of the scFv protein. A protein concentration of the purified scFv protein was determined with Protein Assay kit (BIO-RAD).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com