vWF (von willebrand factor) activity protection fluid
A protective solution and active technology, which is applied in the fields of peptides, animal/human proteins, organic chemistry, etc., can solve the problems of poor therapeutic pertinence, long antibody preparation cycle, and low purity, so as to avoid heterogeneous antigen rejection and improve The effect of safety in clinical use and reduction of manpower and time costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
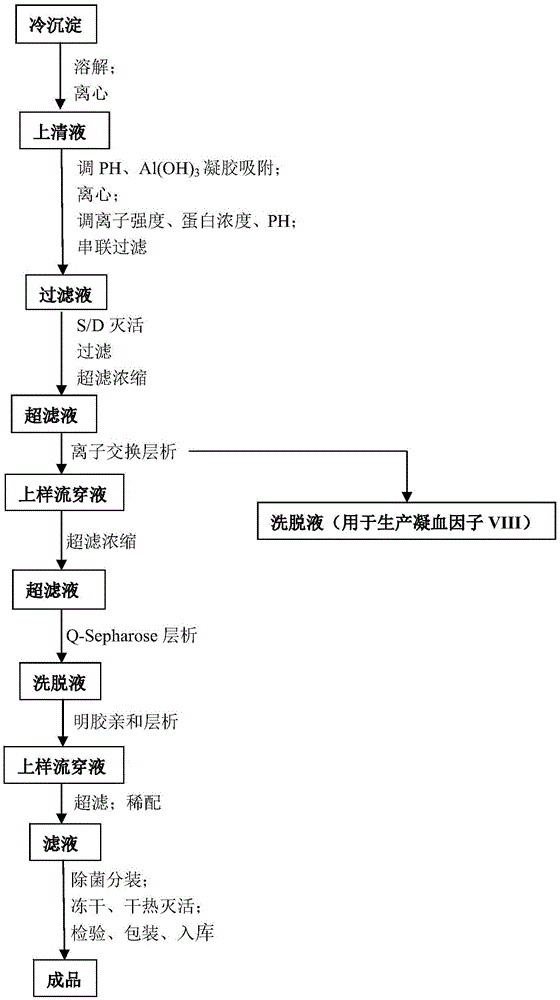
[0048] Taking 6000L plasma as an example, the specific preparation process is as follows:
[0049] (1) During the quarantine period, after receiving the plasma from qualified individuals, wipe the surface of the plasma bag with 75% ethanol, rinse it with water for injection, merge it into a slurry tank, and melt it with circulating water below 30-35°C. The temperature of the plasma should not be higher than 4°C. ℃; after melting, centrifuge, control the liquid temperature at 0-4 ℃, and collect 45.2kg of cryoprecipitate;
[0050] (2) Add the cryoprecipitate obtained in step (1) into 3IU / ml heparin sodium solution, stir until the cryoprecipitate is completely dissolved, and control the temperature of the circulating water at 28-37°C; start centrifugation, collect the supernatant, weigh Weighs 169.5kg;
[0051] (3) Adjust the pH of the supernatant obtained in step (2) to 6.0-7.0 with 1mol / L HCL; add 2% aluminum hydroxide gel, stir; start centrifugation, collect the supernatant, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com