Recombinant blood coagulation factor VIII and application thereof
A technology for coagulation factors and recombinant cells, applied in the directions of coagulation/fibrinolysis factor, factor VII, application, etc., can solve the problems of low protein secretion and function, low transfection efficiency of F8 virus vector, production of antibodies and inhibitor reactions, etc. Achieve low antibody response, improve transfection efficiency and expression efficiency, and correct bleeding phenotype
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] This embodiment provides a method for constructing a lentiviral vector, which specifically includes the following steps:
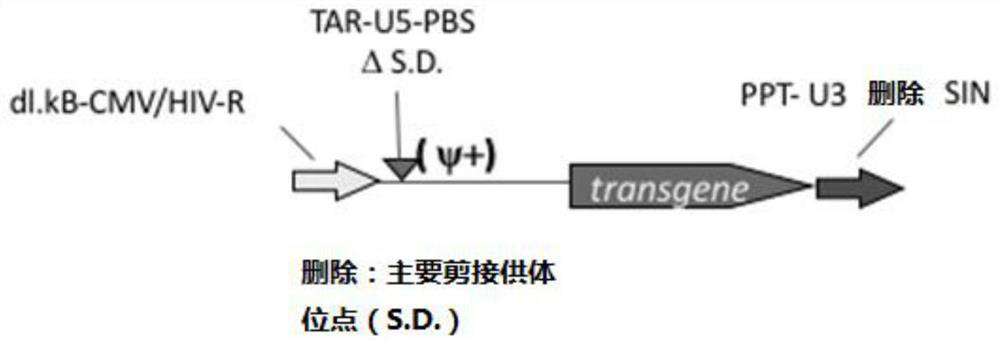
[0074] (1) Schematic diagram of the structure of the lentiviral vector pEGWI as shown in figure 1 As shown, the wild-type 5' splice donor site GT is mutated to CA, the enhancer in U3 is deleted, and a silencer (CH4 silencer) is added in U3. For the specific transformation method, please refer to "Contributions of Viral Splice Sites and cis -Regulatory Elements to Lentivirus Vector Function, Cui et al. Journal of Virology, July 1999, p.6171–6176”;
[0075] (2) Insertion of promoter and F8-BDD, F8-BDD-N8 or F8-BDD-299 gene:
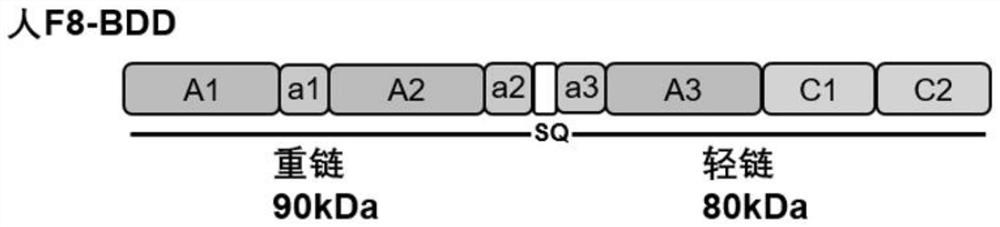
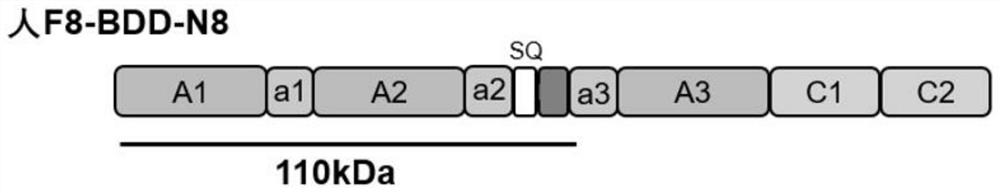
[0076] Chemically synthesize normal F8-BDD (sequence shown in SEQ ID NO.7), F8-BDD-N8 (sequence shown in SEQ ID NO.5) through the entire gene and select possible glycosylation site sequences ), F8-BDD-299 (sequence shown in SEQ ID NO.6) gene sequence, and human EF1α promoter sequence added; normal F8-BDD gene structure diagram ...
Embodiment 2
[0080]In this example, the lentiviral vector constructed in Example 1 was further packaged, purified, and concentrated to obtain the recombinant lentivirus. For the experimental method, refer to ([1] Chang L J, Urlacher V, Iwakuma T, et al. Efficacy and safety analyzes of a recombinant human immunodeficiency virus type 1derived vector system[J].Gene Therapy,1999,6(5):715-728.[2]Chang L J,ZaissAK.Chang,LJand Zaiss,AK.Lentiviral vectors.Preparation and use.Methods Mol Med69 :303-318[J].Methods in molecular medicine, 2002,69:303-318.)
[0081] For specific steps, please refer to the above documents, and a brief description is as follows:
[0082] (1) The lentiviral vector constructed in Example 1 and the packaging helper plasmid pNHP and pHEF-VSV-G were co-transfected into mammalian cells HEK293T and cultured for 48 hours, and the supernatant viral vector was collected;
[0083] (2) purifying and concentrating the lentiviruses collected from the culture to obtain the recombinant...
Embodiment 3
[0086] In this example, the recombinant lentivirus prepared in Example 2 was tested in vitro.
[0087] Three lentiviruses (LV-F8-BDD, LV-F8-BDD-N8 and LV-F8-BDD-N8 and LV-F8-BDD- 299) respectively transfected EA-hy926 endothelial cells, lentiviral transfection methods include:
[0088] Add DMEM medium containing 10% fetal bovine serum and 1% penicillin-streptomycin solution to a six-well plate (Corning, USA), and inoculate 4 × 10 per well. 4 EA-hy926 cells at 37°C, 5% CO 2 Cultured under conditions for 18 h, transfected with lentivirus at MOI=50, and supplemented polybrene (Polybrene, 8 μg / mL, Sigma-Aldrich) to a final volume of 600 μL of the medium, transfected for 24 h, and then replaced with fresh medium every day, When the confluence of the cells reaches 90%, transfer the cells to T75cm 2 Culture flask (Corning, USA).
[0089] Perform protein expression detection to clarify the expression of F8-BDD, F8-BDD-N8 and F8-BDD-299 genes in cells. The specific process is as fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com