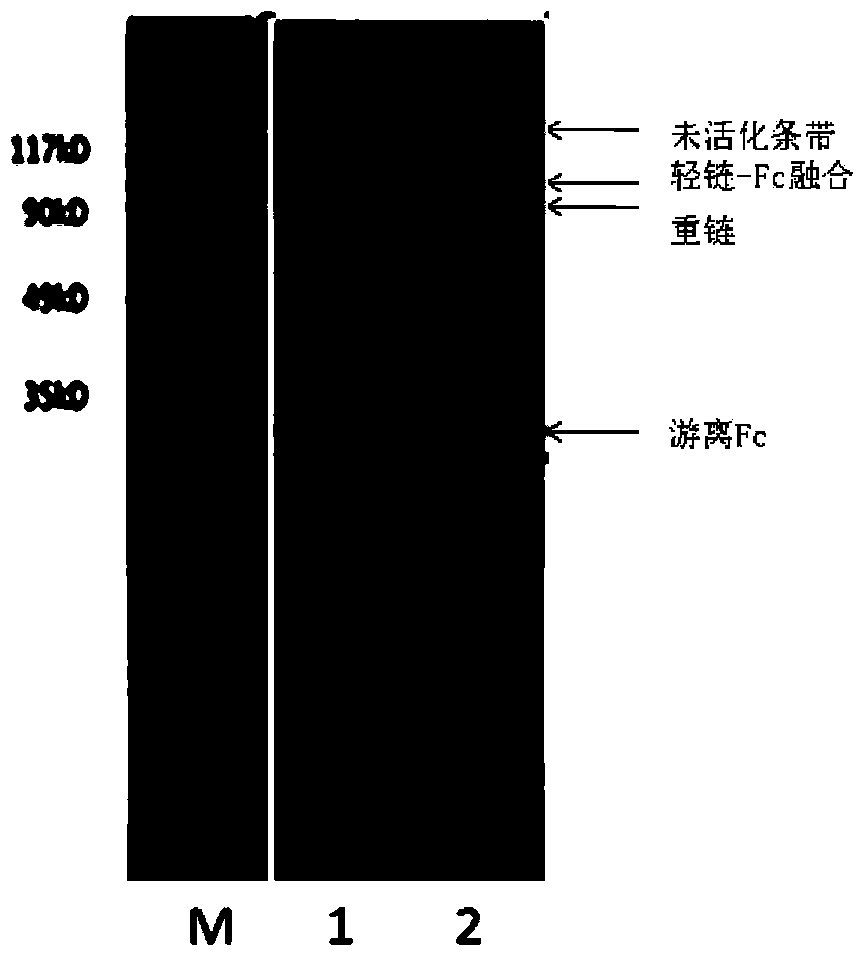
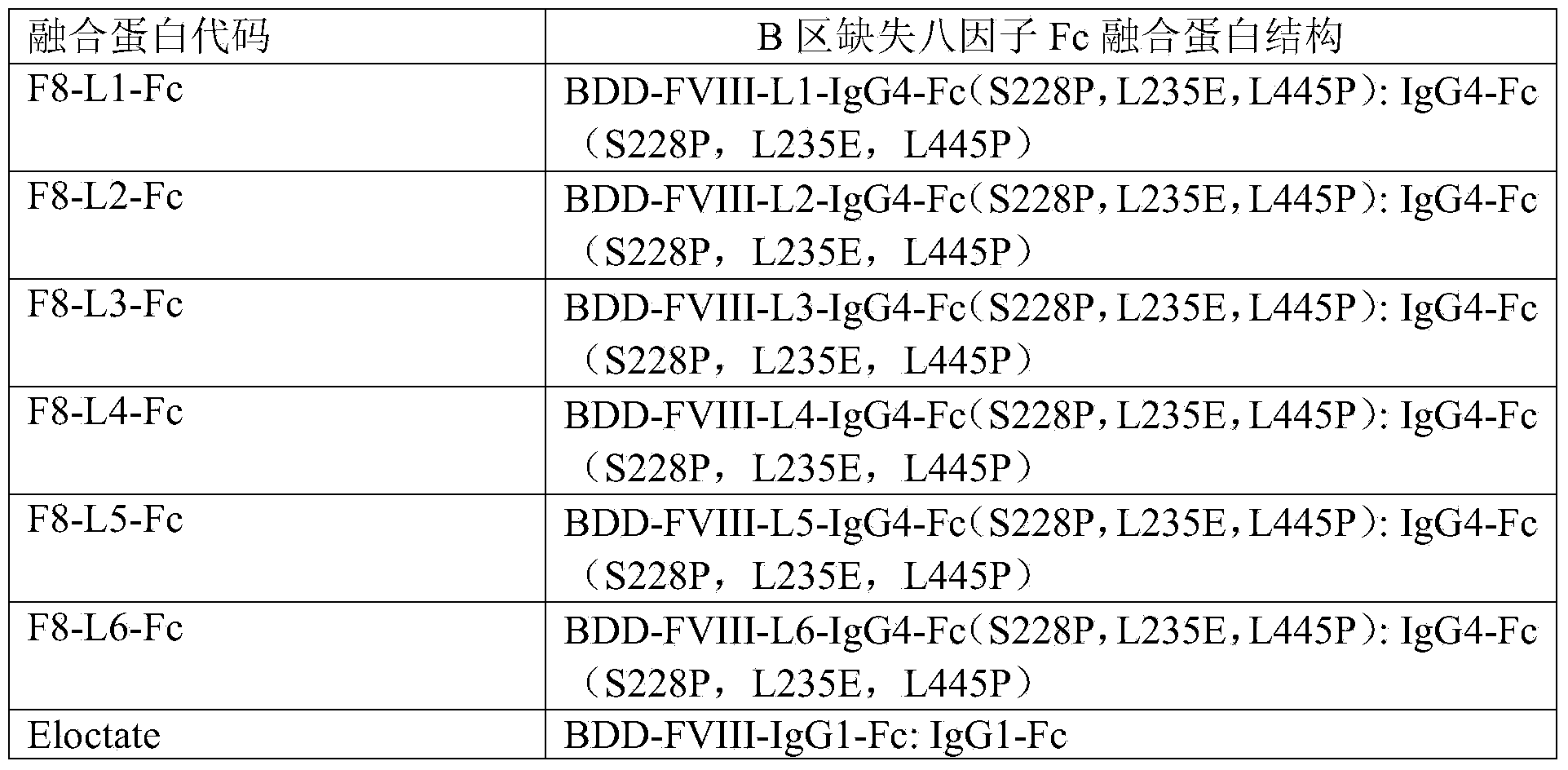
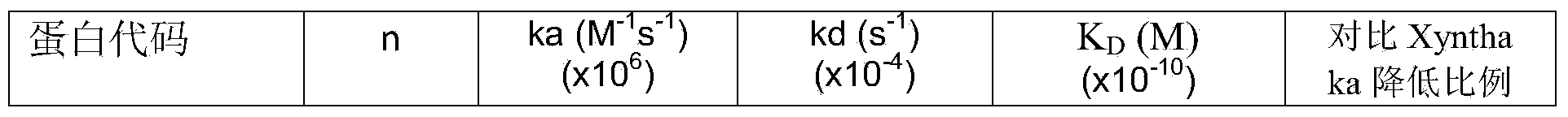Blood coagulation factor VIII fusion protein as well as preparation method and use thereof
A technology of eight coagulation factors and fusion protein, which is applied in the field of eight coagulation factors fusion protein and its preparation to achieve the effect of reducing ADCC effect function and improving in vitro biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1 obtains the fusion gene sequence of coding fusion protein
[0085] The six fusion proteins F8-L1-Fc, F8-L2-Fc, F8-L3-Fc, F8-L4-Fc, F8-L5-Fc, and F8-L6-Fc corresponding to each of the six fusion proteins of the present invention were obtained as follows: Gene sequence encoding DNA:
[0086] Using the cDNA missing factor 8 in region B as a template, the gene sequence of factor 8 missing in region B was amplified by PCR (and the translation stop codon at the C-terminus of the gene sequence missing factor 8 in region B was mutated into BamHI). Points are XbaI, BamHI. The upstream primer is tggtgaactctctagaccca (SEQ ID NO. 21), and the downstream primer is tggtgaactctctagaccca (SEQ ID NO. 22).
[0087] Using IgG4 cDNA as a template, the IgG4 Fc gene sequence containing different connecting peptides was obtained by PCR amplification, and the restriction sites at both ends were BamHI and MluI. in,
[0088] The upstream primer for amplifying the IgG4 Fc gene se...
Embodiment 2
[0104] Example 2 Construction of expression vector
[0105] The invention adopts the double-plasmid co-transfection method to introduce the fusion gene encoding the fusion protein into the host cell for expression. Therefore, a dual-plasmid expression vector was constructed first.
[0106] The specific method is: the eight-factor Fc fusion protein coding sequence (fusion gene) obtained in Example 1 is cloned simultaneously into two pIRES-DHFR (abbreviated as pID) expression vectors respectively, and the cloning sites used are respectively NheI and MluI. The first plasmid expresses a simple Fc sequence under the drive of the CMV promoter (that is, the Fc sequence without the deletion of eight factors in the B region), that is, the IgG1-Fc of Eloctate or the IgG4-Fc of each fusion protein of the present invention (S228P, L235E, L445P ) sequence to construct the pID-Fc expression vector; the second plasmid is also driven by the CMV promoter to express the Fc sequence containing...
Embodiment 3
[0107] Example 3 Establishment of CHO Engineering Cell Line
[0108] The above expression vectors were amplified using Escherichia coli. After the plasmid was extracted, the two expression vectors pID-Fc and pID-8Fc were co-transfected into CHO-DXB11 cells by liposome transfection method for expression.
[0109] The specific method is: in a 6-well plate, CHO-DXB11 blank cells are passaged into a 6-well plate at a ratio of 1:10. Since the CHO-DXB11 cells are DHFR gene-deleted, DMEM medium containing 10% FBS and 1xHT is required for the process. nourish. Transfection experiments were performed when the cell confluency reached 90%. First remove the cell culture medium and add 2 ml of fresh FBS+HT medium to each well. Mix 2 micrograms of pID-Fc plasmid with 12 micrograms of pID-8Fc plasmid, then dilute the obtained 14 micrograms of mixed plasmid DNA to 150 microliters of DMEM medium, and dilute 12 microliters of Lipofectamine reagent to 150 microliters of DMEM medium. Plasmid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com