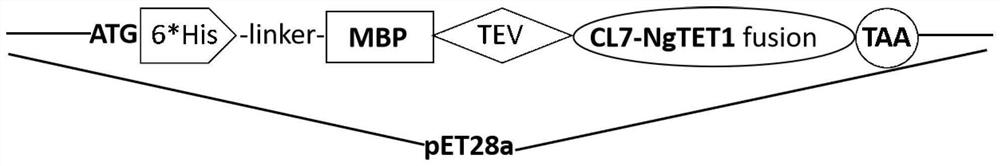
CL7 protein, high-activity recombinant TET enzyme CL7-NgTET1, prokaryotic expression vector and application thereof
A prokaryotic expression, cl7-ngtet1 technology, applied in the biological field, can solve the problems of limited application prospects, low accuracy, and low efficiency of NgTET1, and achieve the effects of improving biological activity in vitro, improving oxidation capacity, and solving low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Construction of recombinant CL7-NgTET1 vector
[0026] (1) Gene synthesis: synthesize according to the protein sequences of SEQ ID NO.1, SEQ ID NO.2, and SEQ ID NO.3 as templates;
[0027] (2) Primer design: According to the two ends of the above fragments and the two ends of the pET28a vector entry point, about 20 bp respectively, the amplification and assembly primers were designed as homology arms. The primer sequences are as follows:
[0028] M-Forward: 5'-GTGCCGCGCGGCAGCCATATGAAAATCGAAGAAGGT-3',
[0029] M-Reverse: 5'-CGGTTCGTTAGATTTGGAACCCTGAAAATACAGATT-3',
[0030] CN-Forward: 5'-AATCTGTATTTTCAGGGTTCCAAAATCTAACGAACCG-3'
[0031] CN-Reverse: 5'-TGCTCGAGTGCGGCCGCTTATTTGGTTTCTTTATGA-3'
[0032] V-Forward: 5'-ATAAGCGGCCGCACTCGAGCA-3'
[0033] V-Reverse: 5'-ACCTTCTTCGATTTTCATATGG-3'
[0034] (3) Gene amplification and gel recovery: Use the KOD high-fidelity DNA polymerase amplification system, add the above primers and templates to form a 50uL reactio...
Embodiment 2
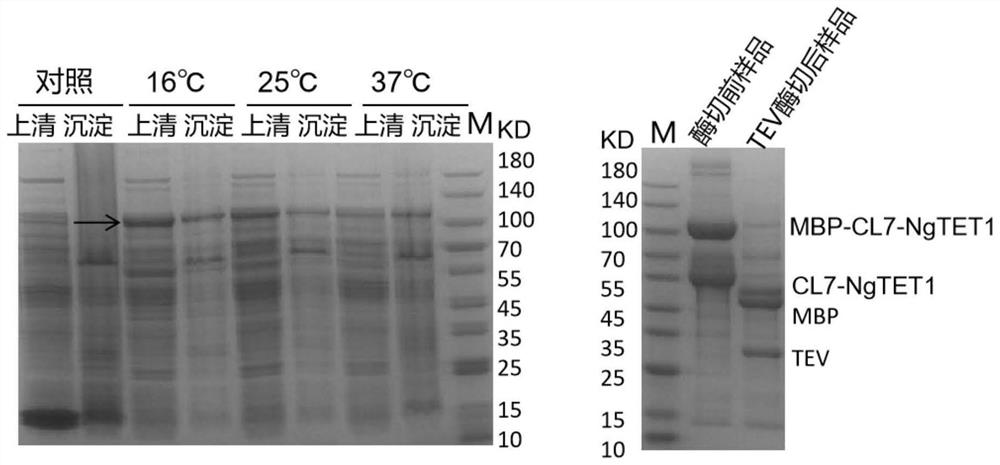
[0038] Example 2: SDS-PAGE and enzyme digestion verification of the expression of recombinant highly active CL7-NgTET1 in Rossetta (DE3)
[0039] The specific implementation steps are as follows:
[0040] (1) Conversion: From Take out Rossetta (DE3) competent cells in the ultra-low temperature refrigerator Thaw on ice and add ligation product keep on ice Heat shock at 42°C in a water bath Place on ice again join in No anti-LB liquid medium at room temperature, placed in a shaker at 37°C for 60 min at a speed of 180r / min; mix the obtained bacterial liquid and spread it to a concentration of On the kanamycin-resistant LB plate, the plate was inverted and cultured at 37°C until monoclonal plaques were formed;
[0041] (2) Induction: Pick four single clones and inoculate them here contain In the LB test tube of kanamycin, shake culture at 250r / min in a shaker at 37°C, when the OD600 reaches 0.1-1.0mM isopropyl-β-D-thiogalactoside was added to three of the test...
Embodiment 3
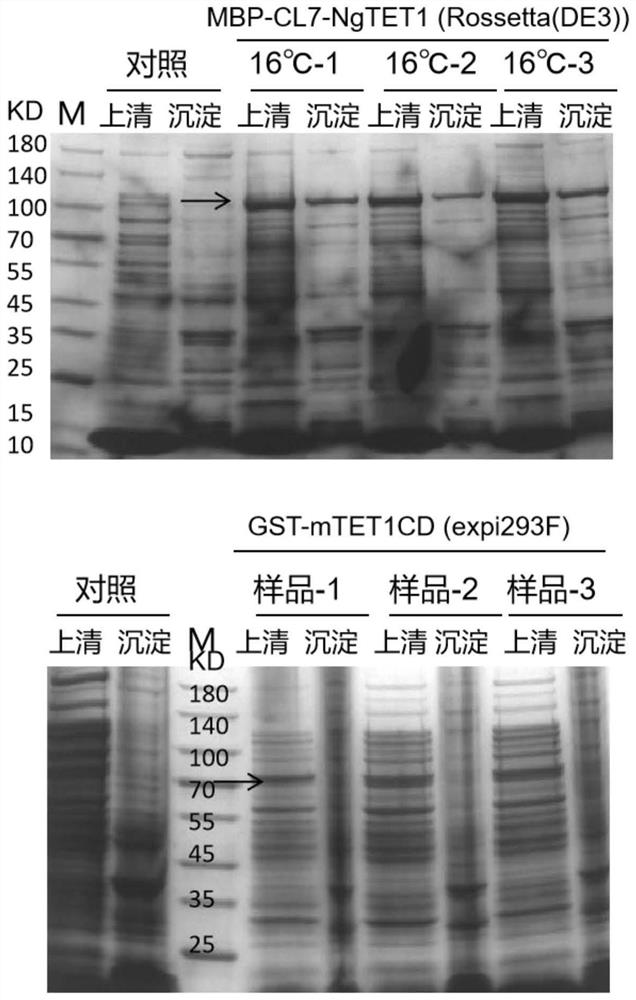
[0049] Example 3: Comparison of expression levels of recombinant CL7-NgTET1 prokaryotic and mTET1CD eukaryotic series.
[0050] In this embodiment, the expression level of CL7-NgTET1 in E. coli Rossetta strain and the expression level of mTET in 293 cells were analyzed by SDS-PAGE and BCA, and then compared.
[0051] The expression operation of recombinant CL7-NgTET1 in prokaryotic is the same as that in the above-mentioned Example 2. Four groups of samples were taken to run the gel, one group was a blank control, and the other three groups were repeated under 16°C induction conditions, and the supernatant and precipitate were separated for SDS-PAGE.
[0052] The expression operation of GST-mTET in 293 cells is as follows:
[0053] (1) Plasmid and cell preparation: The N-terminal of mTET1CD was fused with GST and constructed into pEE12.4 expression vector to form pEE12.4-GST-mTET1CD plasmid, and the pEE12.4-GST-mTET1CD plasmid was amplified with Escherichia coli DH5α , extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com