Arthralgia relief composition, arthralgia relief health-care product, and application of arthralgia relief composition
A joint pain and composition technology, which is applied in the field of joint pain relief composition, can solve the problems of no anti-inflammatory effect, fast relief of limited joints, etc., and achieve the effect of promoting the improvement of joint activities, relieving joint pain, and relieving pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
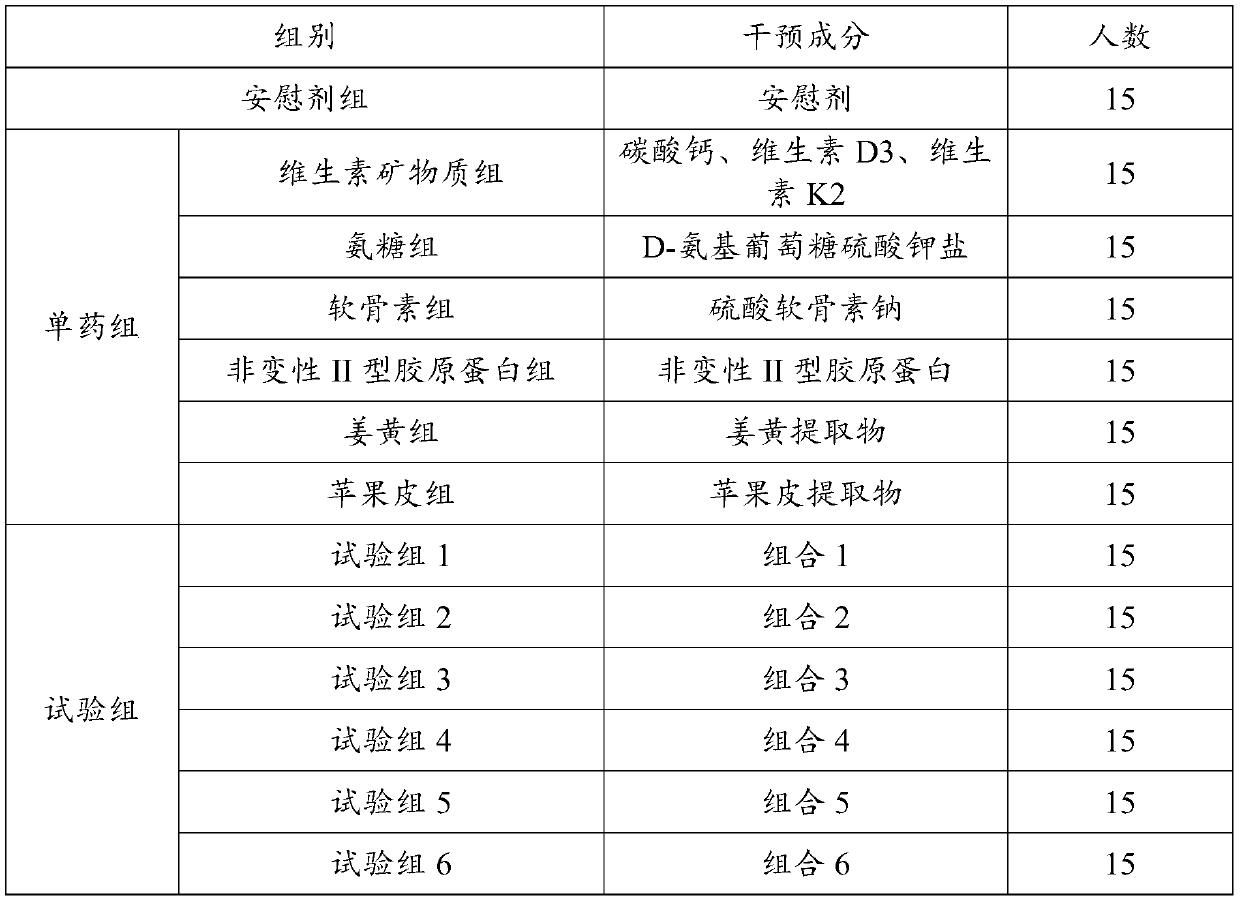
[0030] Embodiment 1: efficacy test
[0031] 1.1 The test groups are shown in Table 1 and Table 2:
[0032] Table 1: Combination 1 ~ Combination 5 formulations
[0033] Composition combination 1 combination 2 combination 3 combination 4 combination 5 combination 6 D-glucosamine sulfate potassium salt (parts) 400 400 Chondroitin Sulfate (parts) 100 100 100 Type II collagen (parts) 50 50 50 50 50 Undenatured Type II Collagen (serving) 10 10 10 10 10 10 Vitamin K2 Powder 10 10 10 Vitamin D3 Powder 1 1 1 calcium carbonate 150 150 150 Turmeric Extract 50 50 50 50 Apple Peel Extract 75
[0034] Table 2: Experimental group settings and intervention programs
[0035]
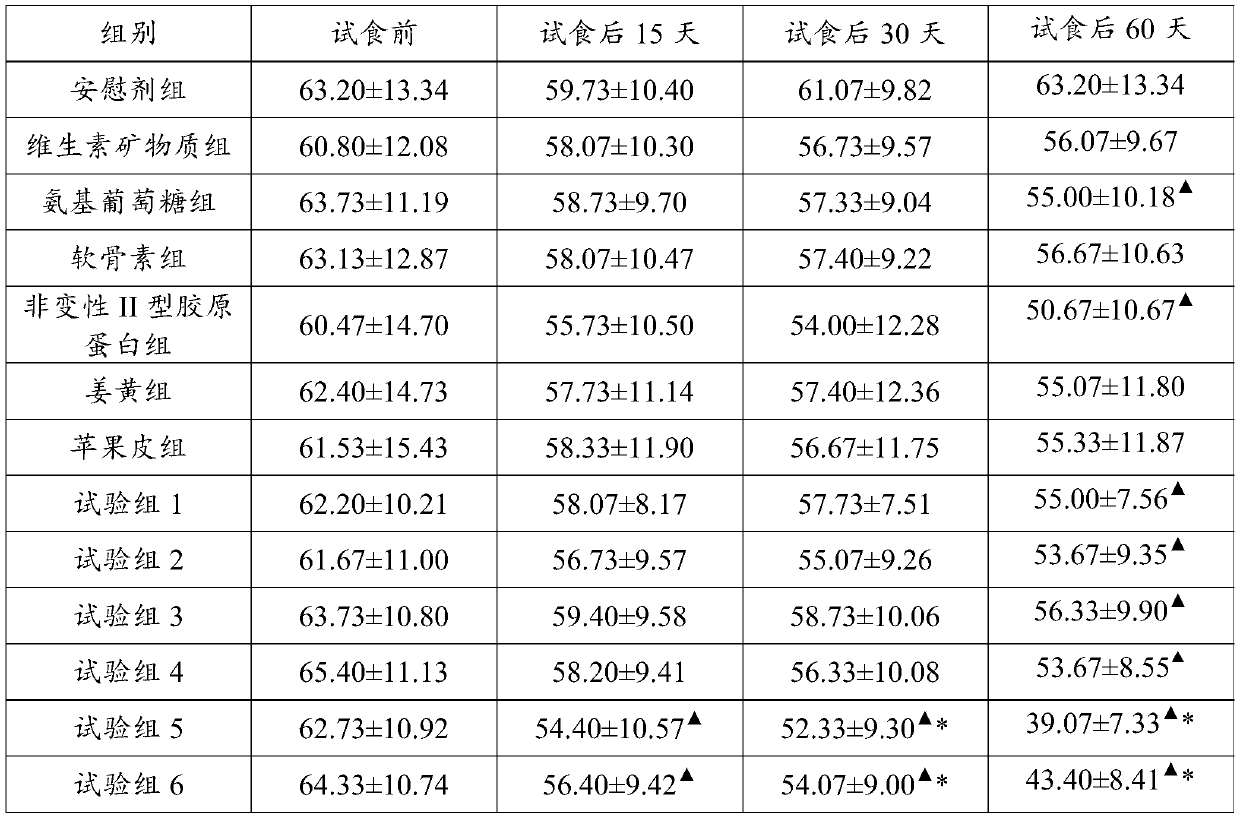
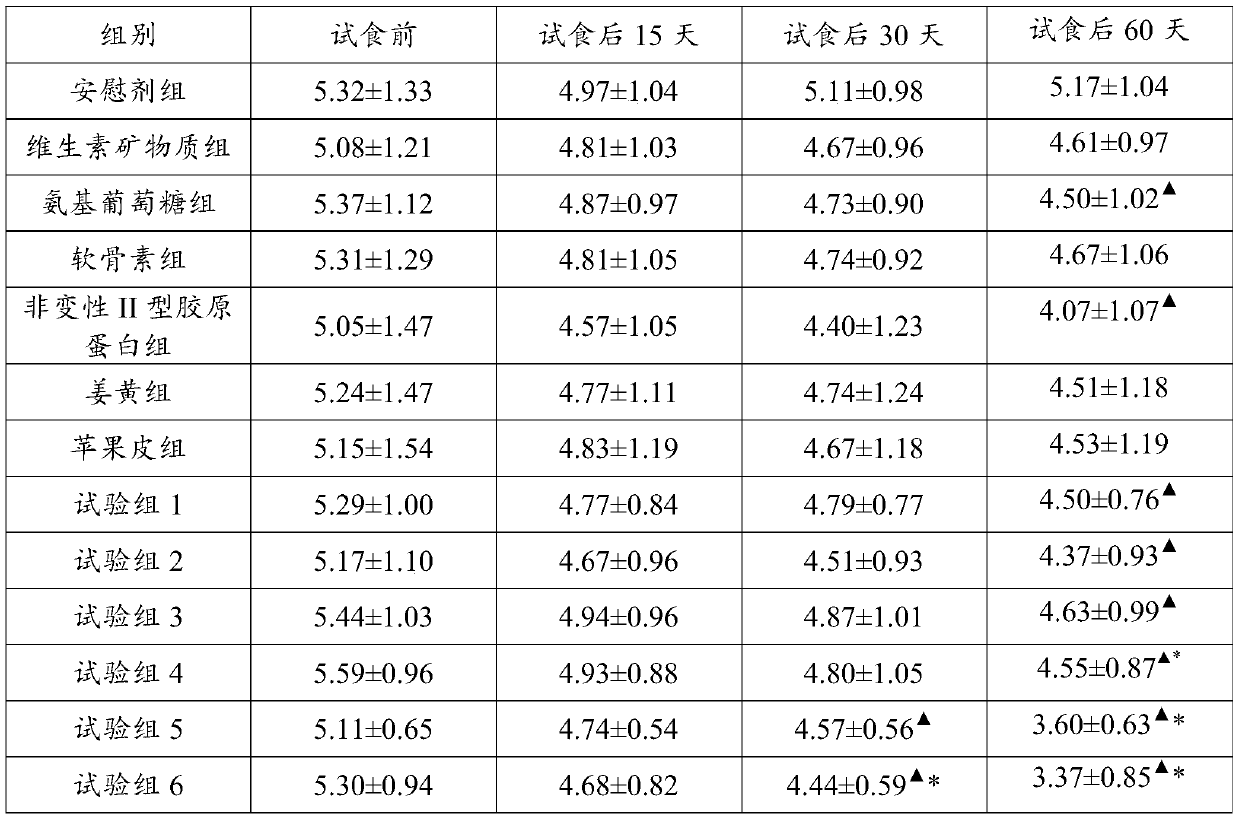
[0036] 2.2 Effect test:
[0037] 2.2.1 Test method
[0038] A total of 195 outpatients with knee arthritis and osteoarthritis were selected and randomized into 13 groups ...
Embodiment 2
[0049] Example 2: Preparation of film-coated tablets
[0050] 1. Product formula
[0051] raw material name unit Dosage (per 1000 tablets) D-glucosamine sulfate potassium salt g 400 calcium carbonate g 150 Chondroitin Sulfate g 100 Turmeric Extract g 50 type II collagen g 50 Undenatured Type II Collagen g 10 Vitamin K2 Powder g 10 Vitamin D3 Powder g 1 Excipient name unit Dosage (per 1000 tablets) Croscarmellose Sodium g 55 microcrystalline cellulose g 49.92 Film Coating Premix g 33 Copovidone g 28 Magnesium stearate g 12 silica g 10 Carmellose Sodium g 1.08
[0052] 2. The preparation process is as follows:
[0053] 2.1 Weighing and preparing materials:
[0054] Accurately weigh D-glucosamine sulfate potassium salt, calcium carbonate, chondroitin sulfate, turmeric extract, type II collagen, non-denatured type II collagen, vitamin K2 powder, vita...
Embodiment 3
[0065] Embodiment 3: the preparation of capsule
[0066] 1. Product formula
[0067] raw material name unit Dosage (per 1000 capsules) D-glucosamine sulfate potassium salt g 150 calcium carbonate g 50 Chondroitin Sulfate g 50 Turmeric Extract g 50 type II collagen g 50 Undenatured Type II Collagen g 10 Vitamin K2 Powder g 5 Vitamin D3 Powder g 1 Apple Peel Extract g 25 Excipient name unit Dosage (per 1000 capsules) pregelatinized starch g 55.8 Magnesium stearate g 4.5 silica g 4.2 Gelatin Empty Capsules g 1000 capsules
[0068] 2. Preparation process:
[0069] 2.1 Weighing and preparing materials:
[0070] Accurately weigh D-glucosamine sulfate potassium salt, calcium carbonate, chondroitin sulfate, turmeric extract, type II collagen, non-denatured type II collagen, vitamin K2 powder, vitamin D3 powder, pregelatinized starch, Magnesium stearate and silicon d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com