Treatment and prevention of cardiac conditions using two or more isoforms of hepatocyte growth factor
A heart disease, isoform technology, applied in cardiovascular system diseases, medical preparations containing active ingredients, peptide/protein components, etc., can solve coronary artery restenosis, intimal thickening and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Embodiment 1: Construction of plasmid
[0175] The pCK vector can utilize the human cytomegalovirus (HCMV) promoter to drive gene expression and has been described previously (Lee et al., Biochem. Biophys. Res. Commun. 272:230 (2000); WO 2000 / 040737).
[0176] pCK-VEGF165 was constructed by inserting the VEGF165 cDNA into the pCK vector and has been described previously (Lee et al., Biochem. Biophys. Res. Commun. 272:230 (2000); WO 2000 / 040737).
[0177] pCK-cHGF contains the encoded HGF under the control of the HCMV promoter 728 and has been described previously (US 2005 / 0079581).
[0178] pCK-dHGF contains the encoded HGF under the control of the HCMV promoter 723 cDNA and has been described previously (PCT / KR03 / 00548).
[0179] pCK-HGF-X7 contains a hybrid HGF cDNA (SEQ ID NO: 9) designed to express two HGF isoforms simultaneously inserted into the pCK vector and has been described previously (US 2005 / 0079581 ).
Embodiment 2
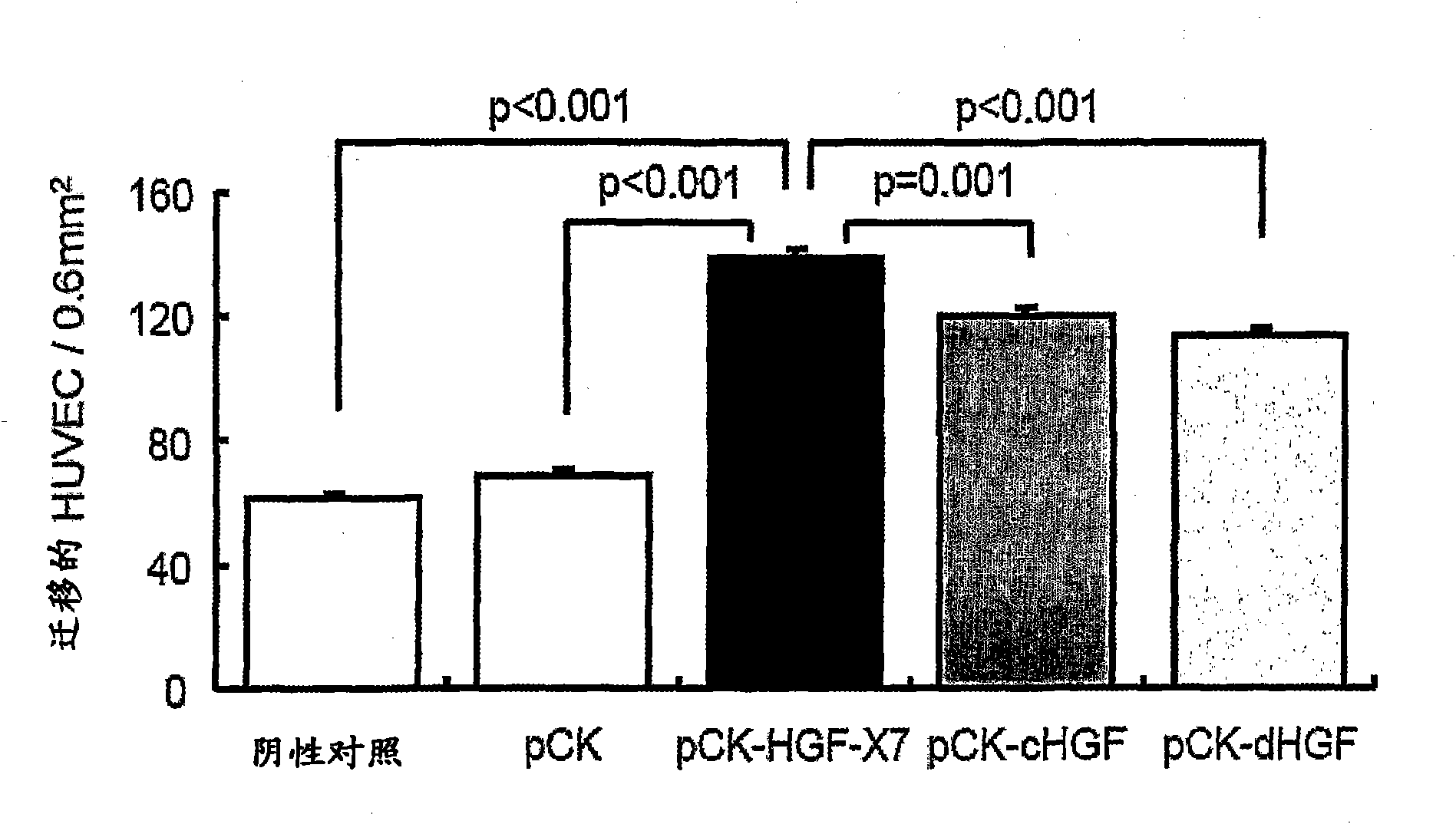
[0180] Example 2: Effects of HGF on Cell Migration and Proliferation
[0181] The purpose of this study was to evaluate the effect of HGF on cell migration and proliferation in vitro.
[0182] 1. Materials and Methods
[0183] (1) Preparation of HGF protein
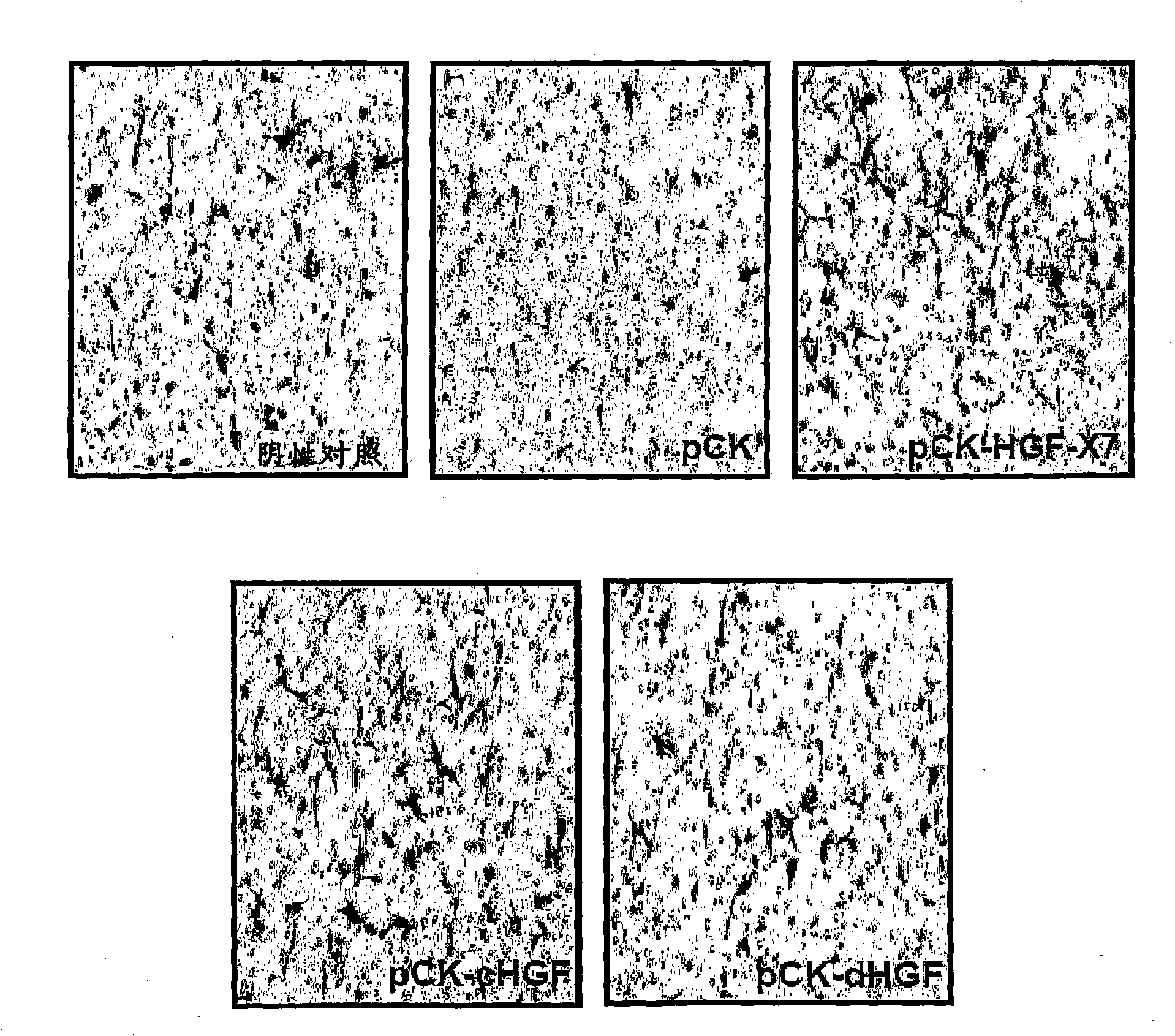
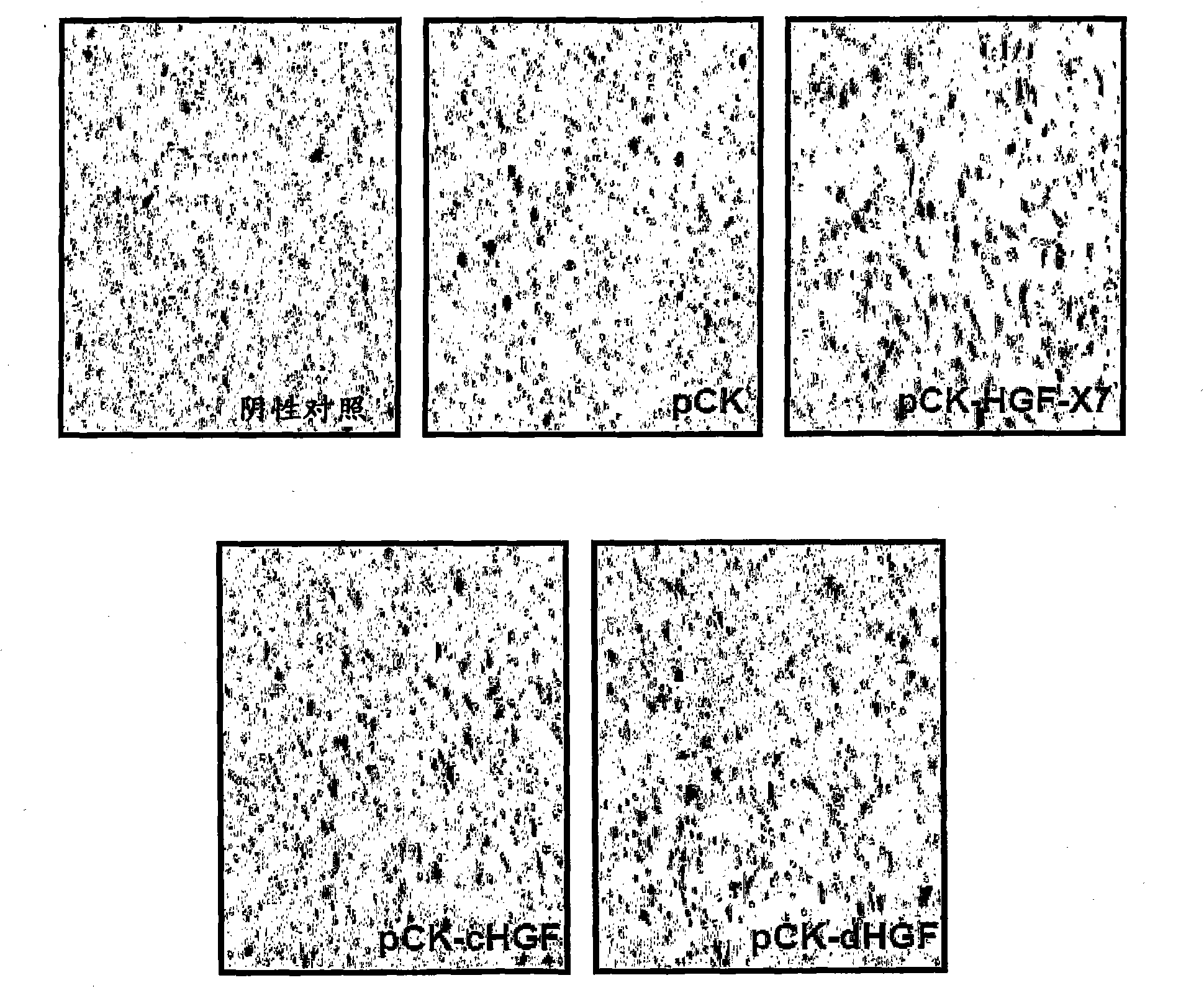
[0184] Using FuGENE6 TM (Roche Diagnostics, Germany) pCK-HGF-X7 was transfected into 293T cells. pCK, pCK-cHGF and pCK-dHGF were used as controls. Two days after transfection, the culture supernatant containing HGF protein was obtained, and the amount of HGF was measured by human HGF ELISA (R&D Systems, MN, USA) according to the manufacturer's recommendation.
[0185] (2) Cell migration assay
[0186] Evaluation of the effect of HGF on human umbilical vein endothelial cells (HUVEC, Agiolab Co., Ltd., ALO1-0122S), mouse skeletal muscle myoblasts (C2C12, ATCC No. CRL-1772) and rats in a modified Boyden chamber assay Effects on migration of cardiac myoblasts (H9C2, ATCC No. CRL-1446). Inserts of 24-well transwell cell...
Embodiment 3
[0193] Example 3: Efficacy Evaluation of HGF in Rat Ischemic Heart Disease Model
[0194] The aim of this study was to evaluate the cardioprotective effect of intramyocardial injection of HGF in a rat model of ischemic heart disease. The experimental steps are shown in Figure 5 middle.
[0195] 1. Materials and Methods
[0196] (1) animals
[0197] Upon arrival, 38 Sprague-Dawley rats (male, 12 weeks old, 350-400 g, SLC) were given food and water ad libitum and allowed to rest for 7 days before surgery.
[0198] (2) Myocardial infarction model
[0199] To analyze the drug effect of HGF in this study, a rat ischemic heart disease model, which is one of the widely used pathological models of CAD, was used. Rats were anesthetized by intramuscular injection of xylazine (5 mg / kg) followed by ketamine (50 mg / kg). After disinfection with 95% alcohol and iodine, the chest was covered with sterile surgical gauze, exposing only the incision area, thereby providing completely ster...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com