Minimally invasive intravascular treatment device
A technology of equipment and blood vessels, applied in the field of catheter-based medical equipment, can solve problems such as failure of deflation, limitations of equipment application fields, and incomplete deflation of airbags
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0185] Shown in the attached drawings is a catheter-based device for the treatment of internal body cavities such as arteries / veins or other hollow organs according to the present invention. Also shown are improved sheaths and sheath loading devices according to the present invention.
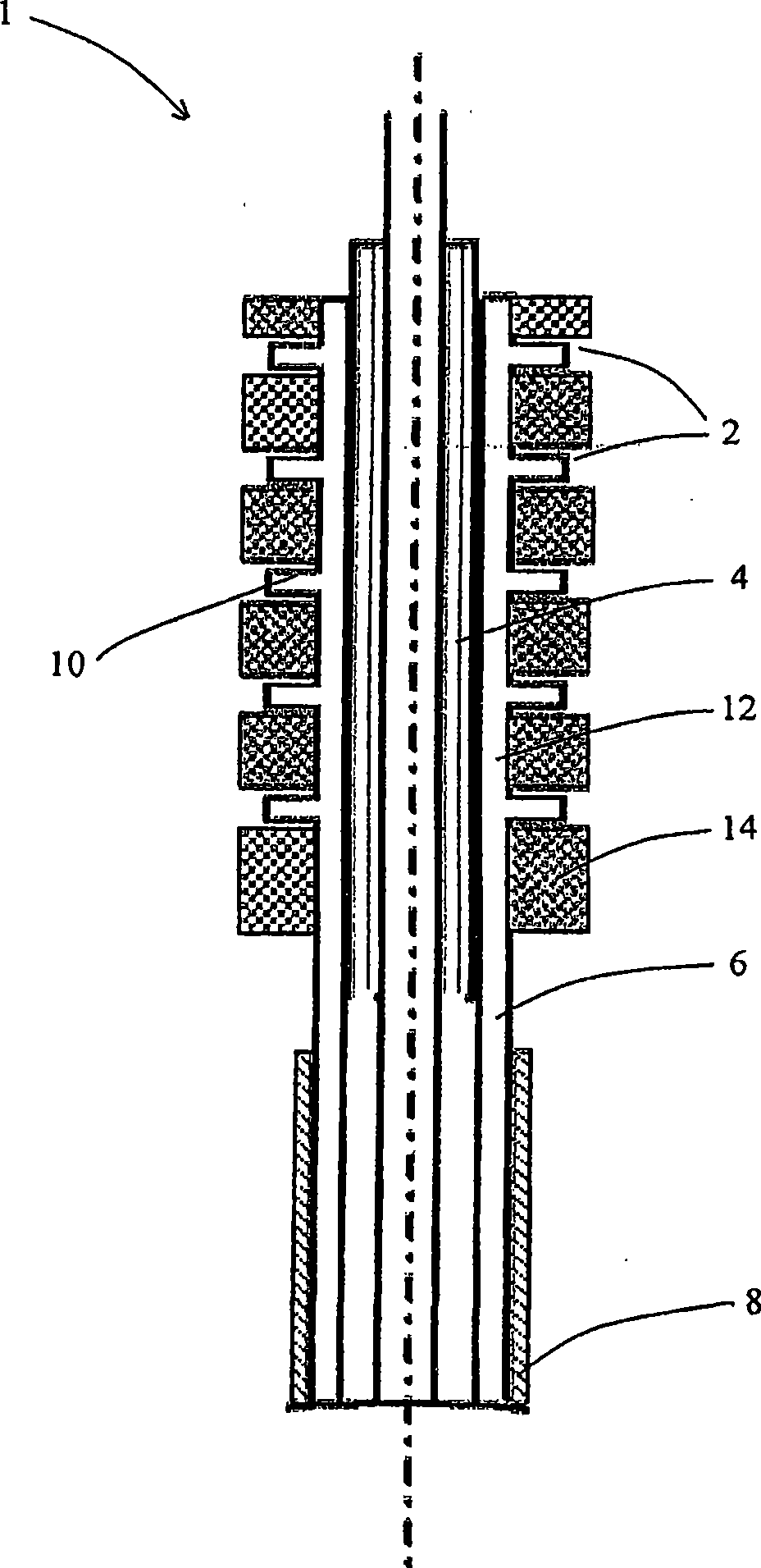
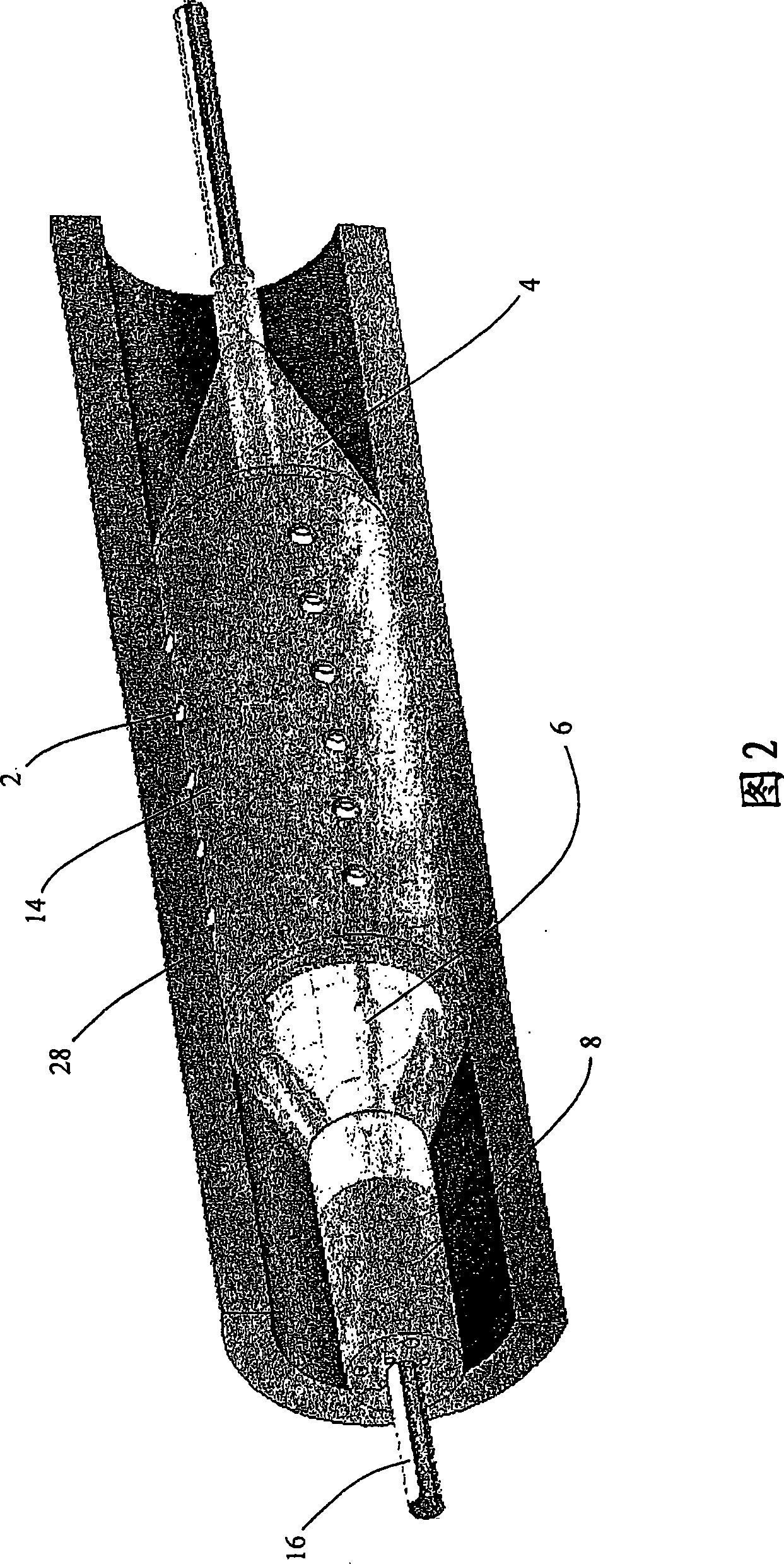
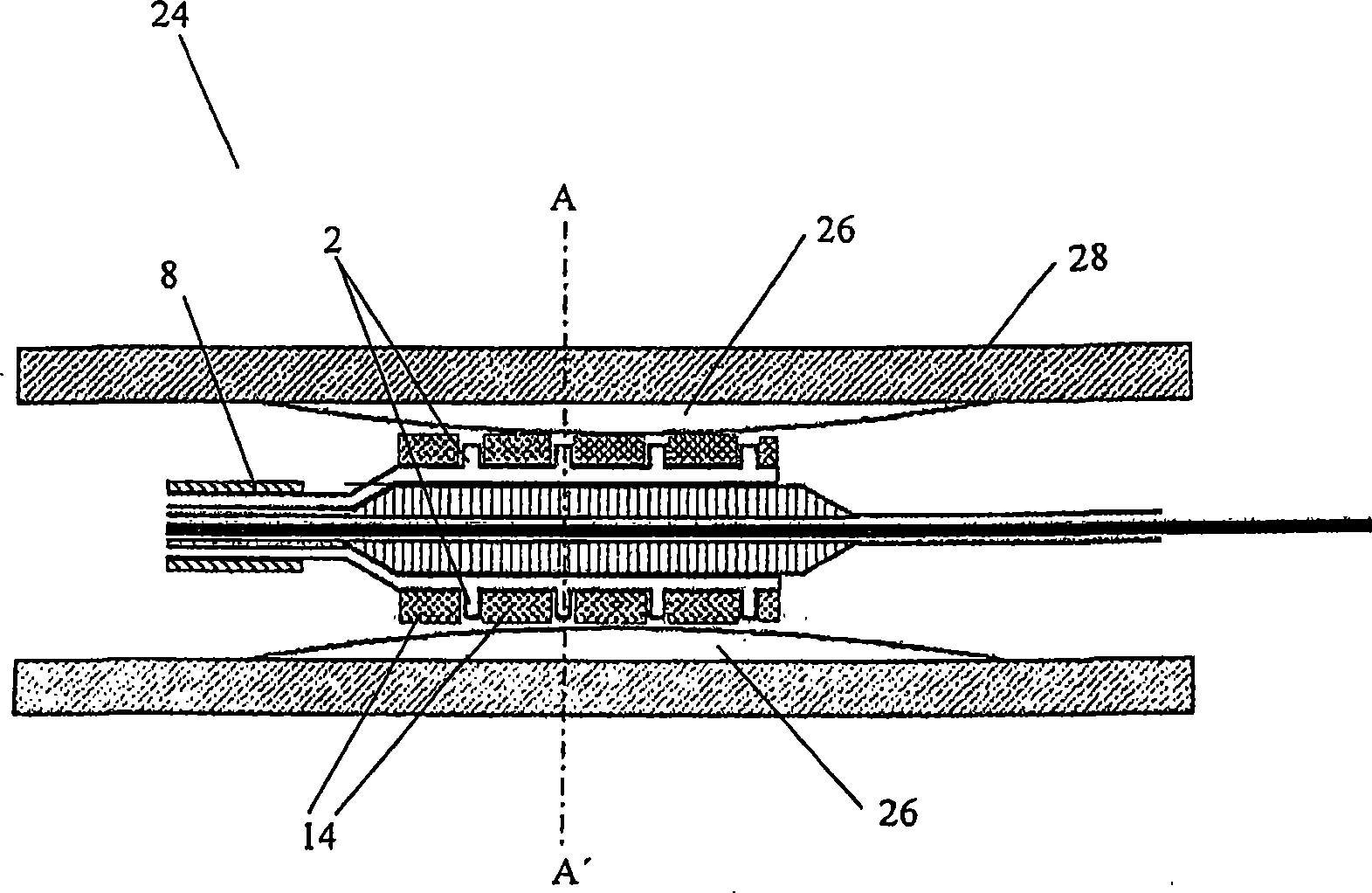
[0186] Attached figure 1 A catheter-based drug delivery device 1 according to an embodiment of the present invention is shown. The device can be inserted into the vasculature through a guide wire (as shown in FIG. 2). The device includes a micro-needle 2, which has two positions, a retracted position and an extended position. These needles are mounted on the surface of the balloon catheter 4 and connected to the multi-lumen supply hose 8 through a flexible tube 6. The needle / microneedle or needle shaft (for direct injection of drugs) is attached to the hollow needle bottom reservoir 12. The needle bar 10 protrudes outward from the reservoir 12 and is protected in a rubber sleeve or sheath 14. Once...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com