Pharmaceutical composition for the prevention or treatment of degenerative neurological brain disorders
一种神经退行性、组合物的技术,应用在药物组合、神经系统疾病、医药配方等方向,能够解决延迟认知功能退化、没有实现等问题,达到抑制活性氧和兴奋性脑神经递质、抑制细胞凋亡、增强记忆力的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
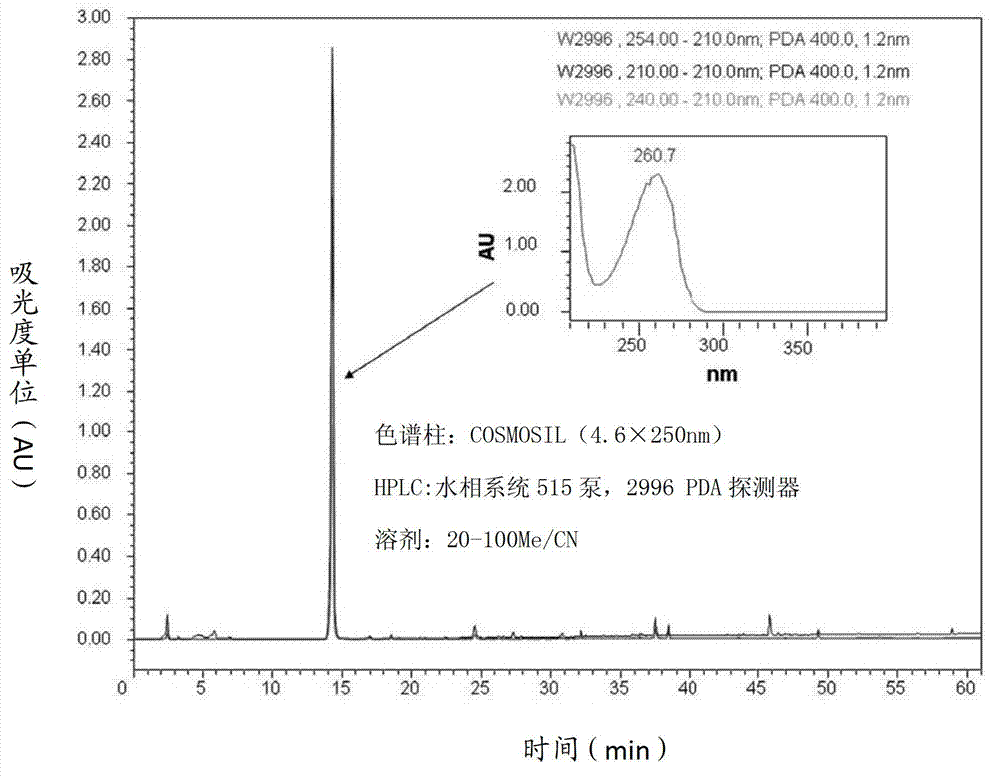
[0062] Embodiment 1: the synthesis of methyl p-hydroxybenzoate
[0063] After reacting phenol and carbon dioxide at 100 atmospheres and 125°C, sulfuric acid is added to produce p-hydroxybenzoic acid. Then use alcohol to esterify the compound, such as methanol to synthesize methyl p-hydroxybenzoate (MP).
[0064] The synthesized methyl p-hydroxybenzoate (MP) was analyzed by high performance liquid chromatography (HPLC), and the results were in figure 1 displayed in .
[0065] In addition, the synthesized methylparaben (MP) was diluted with phosphate buffered saline (PBS) and used for testing and analysis.
Embodiment 2
[0066] Embodiment 2: Hydroxyl radical scavenging ability test
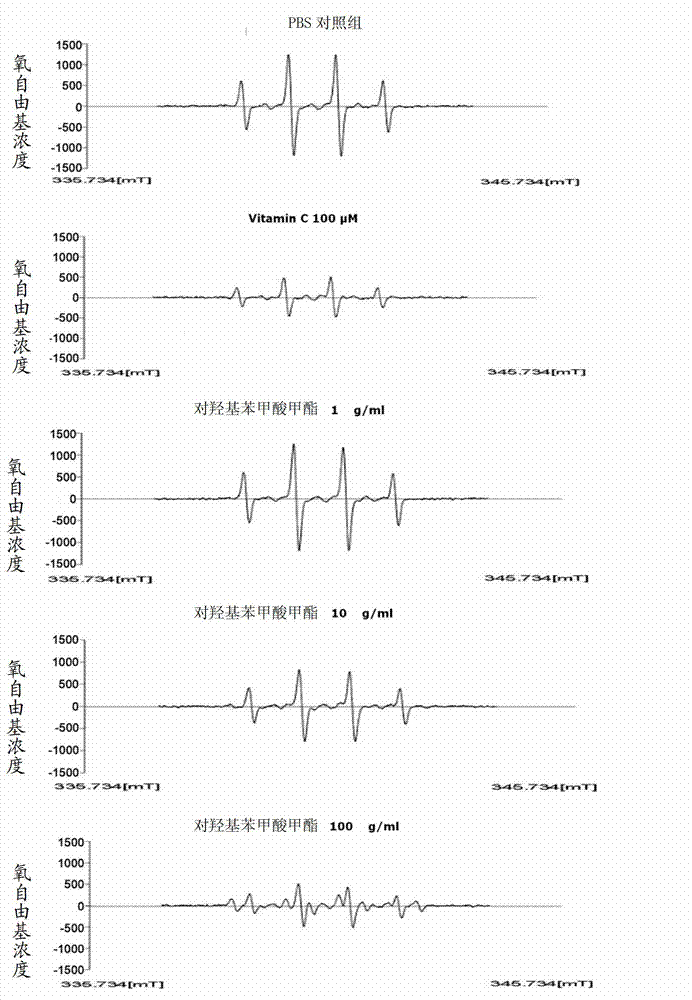
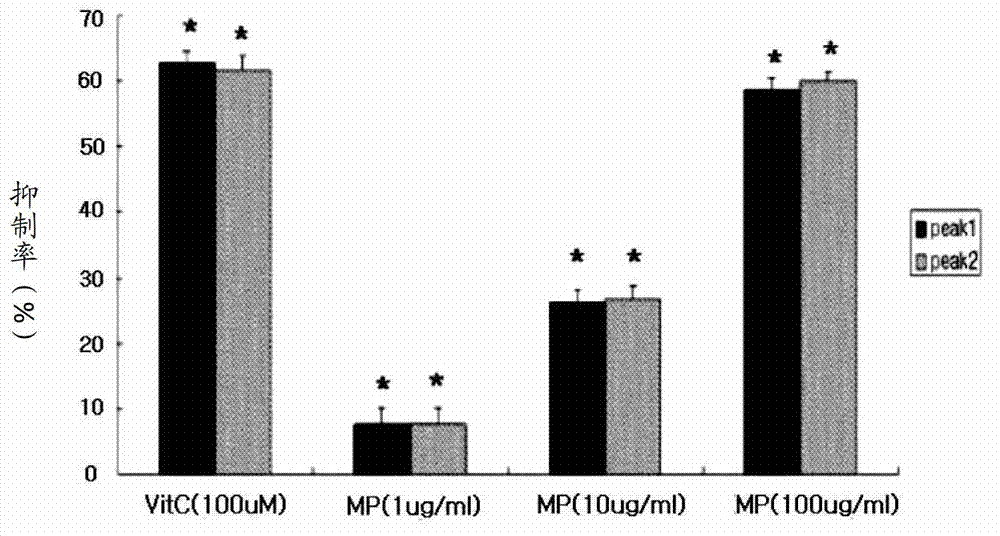
[0067]To identify the ROS removal ability of methylparaben (MP), the Fenton reaction [H 2 o 2 +FeSO 4 ] produce hydroxyl radicals. The generated hydroxyl radicals were captured by nitrone spin trapping agent (DMPO), and the DMPO-OH addition products formed after capture were measured by electron spin resonance (ESR) spectrometer. ESR spectrum by reaction reagent [phosphate buffer solution (pH7.4); 4.5M DMPO10μl, 0.6mM FeSO 4 75μl, 2.8M H 2 o 2 75μl], reacted with methyl p-hydroxybenzoate and vitamin C, and then determined by ESR spectrometer (JES-FA, JEOL, Japan), the measurement results are shown in figure 2 . For this, the ESR spectrometer is analyzed with some parameters [magnetic field 340mT, power 1.0mW, frequency 9.19GHz, amplitude adjustment 0,2mT, gain 200, sweep time 2 minutes, sweep width 10mT, time constant 0.03 seconds, temperature 20°C] .
[0068] Such as figure 2 As shown, from the analys...
Embodiment 3
[0070] Example 3: Testing of Cytoprotective Activity Against Alpha (α)-Synuclein, Reactive Oxygen and Excitatory Brain Neurotransmitters
[0071] To verify whether methylparaben (MP) has cytoprotective effects against reactive oxygen species, excitatory neurotransmitters, and α-synuclein in cultured cells, a cytoprotective ability test was performed. These substances, namely, reactive oxygen species, excitatory neurotransmitters and α-synuclein have been implicated in causing widespread damage to nerve cells associated with Parkinson's and other degenerative brain diseases.
[0072] Each well of the 96-well plate contains SH-SY5Y cells (5×10 3 cells; 10% FBS supplemented with DMEM medium), cultured at 37°C for 24 hours. Then, methyl p-hydroxybenzoate (MP), 50 μM vitamin E (Vit E) or 10 μM MK-801 were added at a concentration of 1, 10 and 100 ng / ml to continue culturing for 4 hours. In addition, a culture solution to which no drug was added was prepared as a control group. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com