Method for determining content and impurity limit of imatinib mesylate based on HPLC-DAD (High performance liquid chromatography-diode array detection) method
A technology for the content of imatinib mesylate, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of chromatographic columns being affected and reducing the service life of chromatographic columns, and achieves simple methods, reduced cost and long service life increased effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of reference substance and test solution for content determination: Take 25 mg of imatinib mesylate reference substance and sample respectively in a 50 mL volumetric flask, dissolve with methanol, dilute to the mark with solvent, and shake well. Take 1mL each and place in a 10mL volumetric flask, dilute to the mark with solvent, and shake well.
[0051] Preparation of test solution for related substance inspection: Take imatinib mesylate sample 25mg and place it in a 50mL volumetric flask, dissolve it with methanol, dilute the solvent to the mark, and shake up.
[0052] Preparation of reference substance solution for related substance inspection: Take imatinib mesylate related substance inspection test solution 1mL in a 100mL volumetric flask, dilute to the mark with a solvent, and shake well.
Embodiment 2
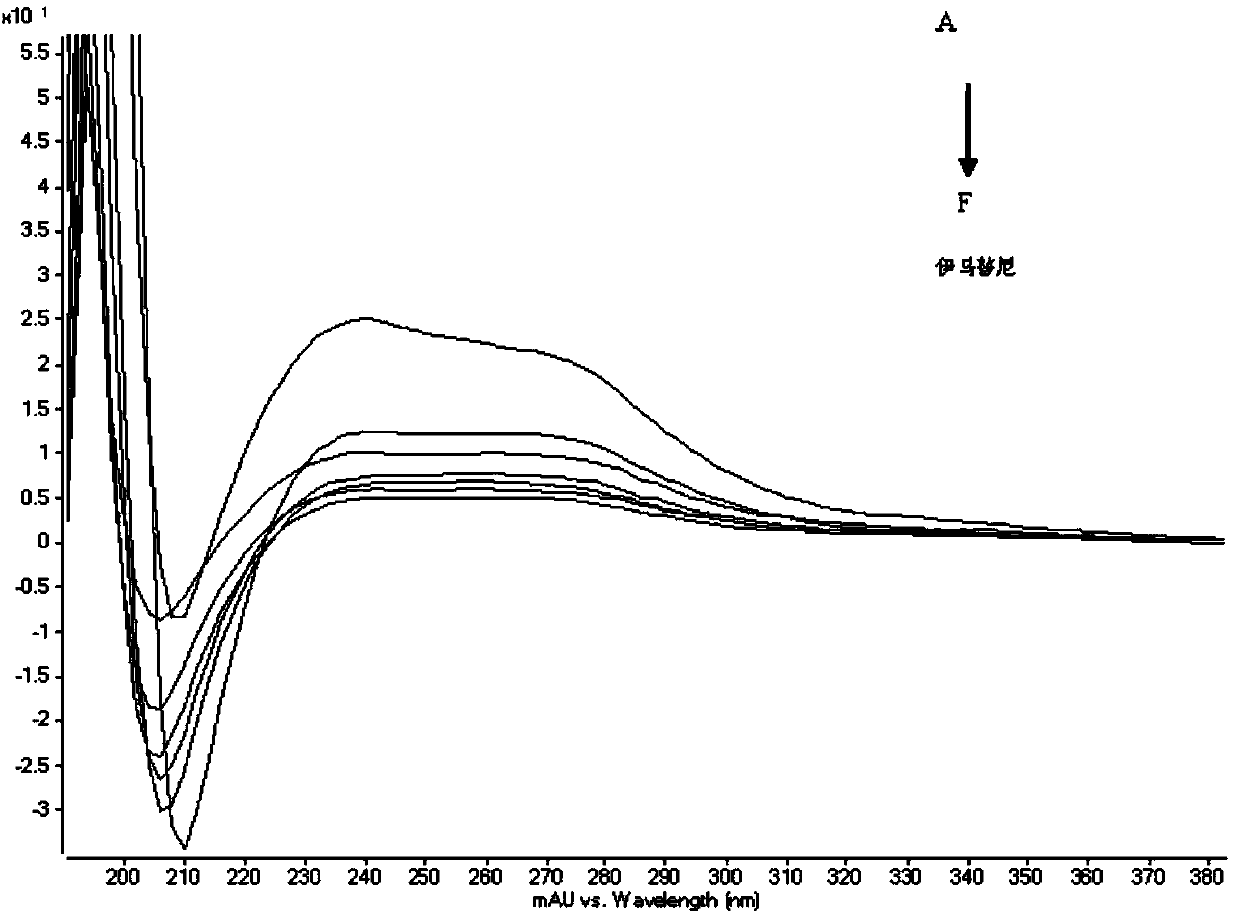
[0054] Get the imatinib mesylate reference substance and prepare the imatinib mesylate solution according to the method of Example 1, and get the imatinib mesylate reference substance and a single impurity and prepare the system suitability according to the method of embodiment 3 After testing the solution, accurately measure 10 μL and inject it into the liquid chromatograph, and perform DAD scanning in the range of 200nm to 400nm (see figure 1 ). The results show that imatinib mesylate and various impurities have maximum absorption at 230nm and 268nm, while triethylamine has a certain absorption at 230nm, which significantly increases the baseline noise and reduces the detection sensitivity, so 268nm is selected as the detection wavelength .
Embodiment 3
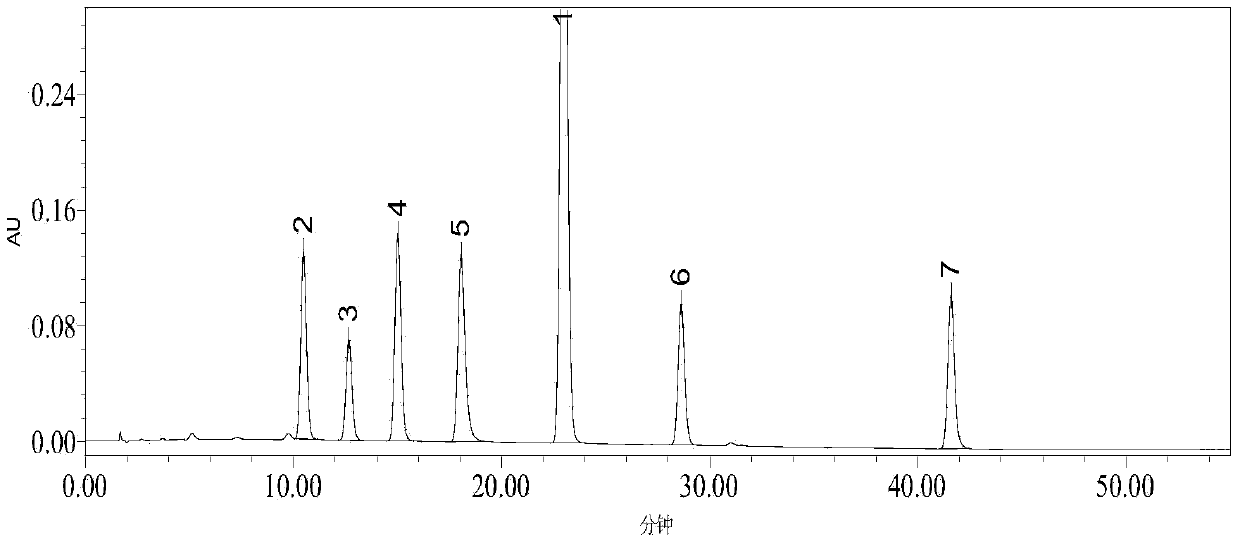
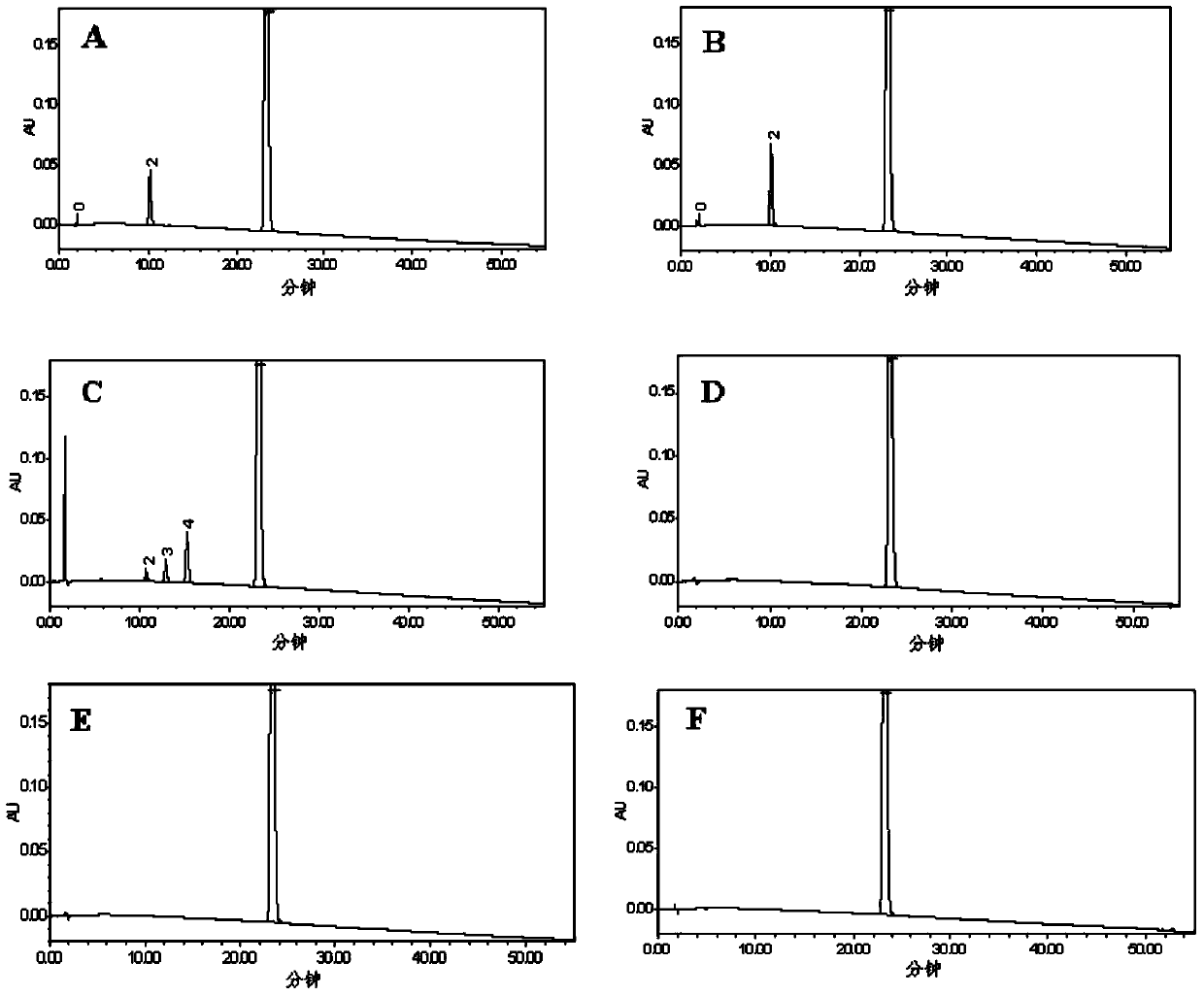
[0056] Take the appropriate amount of imatinib mesylate reference substance, impurity A, impurity B, impurity C, impurity D, impurity E, and impurity F, weigh them accurately, add methanol to ultrasonic treatment to dissolve, and quantitatively dilute with a solvent to make each 1mL A mixed solution containing 0.5 mg of imatinib mesylate and 50 μg of various impurities is used as a system suitability test solution. According to the above chromatographic conditions, 10 μL of sample was injected for analysis, the number of theoretical plates of imatinib mesylate was greater than 10,000, the number of theoretical plates of each impurity was greater than 5,000, the tailing factor was between 0.95 and 1.05, and the components were separated Degrees are greater than 2.0 (see figure 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com