Method for preparing alpha-imatinib mesylate
A technology of imatinib mesylate and imatinib base, applied in the field of medicine, can solve the problems of high cost and high price, and achieve the effects of low production cost, short reaction time and simple operation
Active Publication Date: 2011-09-21
山东安信制药有限公司
View PDF3 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Because the melting point of α-type imatinib mesylate prepared by method (1) is on the low side, and a small amount of β-type products also exist in the α-type imatinib mesylate prepared by method (2), although method (3) The above situation has been improved, but the price of various ketones used in the preparation process is higher, resulting in higher costs in the production process
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a method for preparing alpha-imatinib mesylate, and belongs to the field of medicines. The method comprises the following steps of: suspending imatinib base in a mixed solvent of water and an organic solvent, stirring, and adjusting the temperature to be 20-30DEG C; dissolving methanesulfonic acid, and dripping into reaction liquid; after the dripping is finished, standingfor clarifying the liquid, heating to the temperature of between 50 and 60DEG C, and keeping the temperature to react for 0.5 to 1 hour; and reducing the temperature to 32-40DEG C, adding alpha-imatinib mesylate seed crystals, dripping the organic solvent, continuously reducing the temperature to 20-30DEG C, separating out crystals, filtering, and drying to obtain a product. The preparation method has the advantages of simple operation, short reaction time, high yield, high purity of alpha-imatinib mesylate, low production cost, and suitability for industrial production.
Description
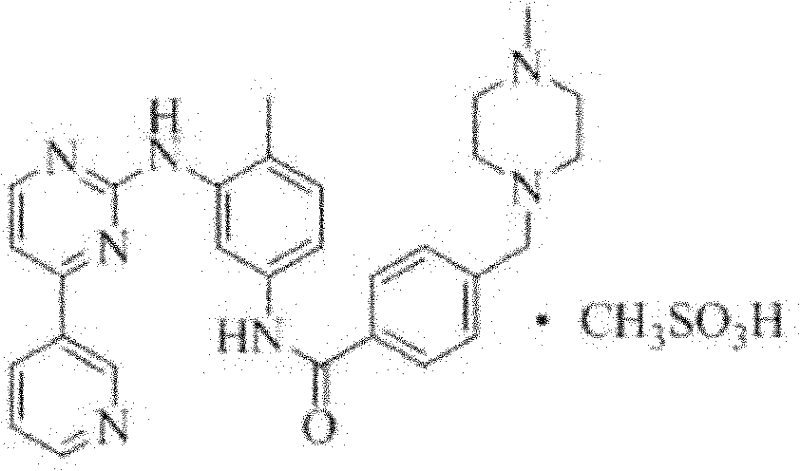
A method for preparing α-type imatinib mesylate technical field The invention relates to a method for preparing α-type imatinib mesylate, which belongs to the field of medicine. Background technique Imatinib Mesylate is a tyrosine kinase inhibitor drug developed by Novartis, Switzerland. Chinese chemical name: 4-[(4-Methyl-1-piperazine)methyl]-N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]phenyl]-Benzene Amide methanesulfonate English chemical name: 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]benzamide, Methanesulfonate Chemical structure: In May 2001, imatinib mesylate was approved by the U.S. FDA for its breakthrough anti-tumor mechanism. For the treatment of myeloid leukemia (CML, Chronic Myebginous Leukemia), the trade name is Glivec; in February 2002, the US FDA approved this product for the treatment of gastrointestinal stromal cell tumors. There are currently 10 FDA-approved indications for imatinib....
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D401/04
Inventor 杨庆坤张雷雷李昌瑜周先国张涛吴柯张兆珍董廷华张玺李江峰卢雪明
Owner 山东安信制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com