Imatinib-containing composition and preparation method thereof
A composition and adhesive technology, which is applied in the directions of medical preparations, drug combinations, and pharmaceutical formulations containing active ingredients, can solve problems such as strong hygroscopicity and difficult preparations, and achieve low cost, good stability, and simple and efficient preparation. The effect of craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
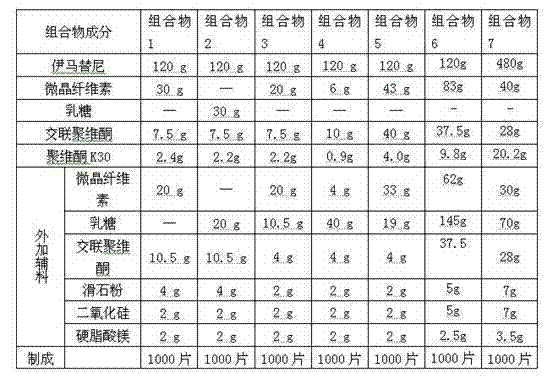
[0038] prescription:
[0039]
[0040] Remarks: the povidone K30 used in composition 1-3 is absolute ethanol solution, the concentration is 3% (w / w), the concentration of povidone K30 absolute ethanol solution used in composition 4 is 1% (w / w) / w), the concentration of povidone K30 dehydrated alcohol used in composition 4-7 is 5% (w / w).
[0041] Preparation method: In addition to povidone, imatinib, microcrystalline cellulose, lactose, crospovidone, talcum powder, silicon dioxide and magnesium stearate are passed through 80-mesh sieve respectively, and set aside.
[0042] Weigh the imatinib of prescription quantity, the microcrystalline cellulose of 4 / 7 total prescription quantity, the crospovidone of 1 / 2 total prescription quantity put fast granulator, mix, add above-mentioned povidone K30 without Hydroalcoholic solution for wet granulation. Dry at about 55°C until the moisture content of the granules is <5%, and sieve the granules with a 24-mesh sieve. Add remaining mi...
Embodiment 2
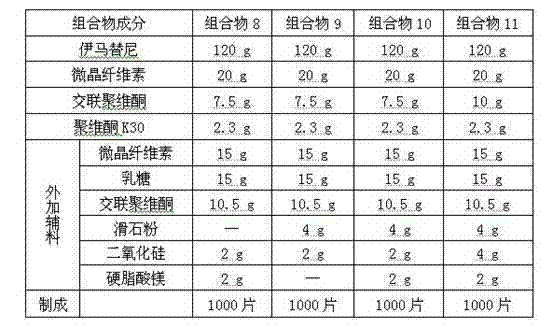
[0049] prescription:
[0050]
[0051] Remarks: The povidone K30 used is an anhydrous ethanol solution with a concentration of 5% (w / w).
[0052] Preparation method: with embodiment 1.
[0053] In this embodiment, the inventors compared the compression molding and fluidity of the compositions when the respective compositions contained different types of lubricants, see compositions 8-10. The results show that the combined use of talcum powder, magnesium stearate and silicon dioxide as lubricants can effectively solve the problems of stickiness and poor fluidity of the composition during the preparation process. The present inventors further conducted a screening test on the dosage of talcum powder, magnesium stearate and silicon dioxide and the disintegrant crospovidone, see composition 11. The result shows that the optimal weight ratio of the lubricating composition lubricant magnesium stearate, talcum powder and micropowder silica gel that the present invention obtains ...
Embodiment 3
[0056] Example 3 pilot test
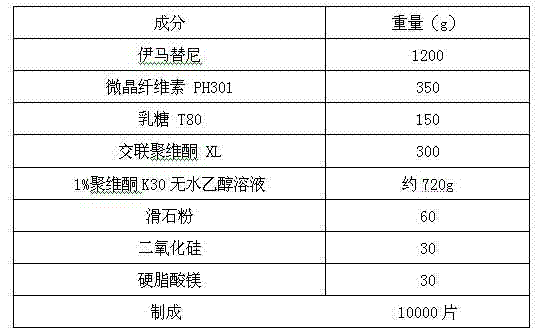
[0057] Prescription:
[0058]
[0059] Preparation Process:
[0060] (1) Preparation and screening of raw and auxiliary materials: except povidone K30, imatinib, microcrystalline cellulose, lactose, crospovidone, talcum powder, silicon dioxide, and magnesium stearate were passed through 80 meshes respectively Sieve and set aside.
[0061] (2) Preparation of povidone K-30 absolute ethanol solution: Weigh an appropriate amount of povidone K-30, add appropriate amount of absolute ethanol, stir to dissolve until clear, add appropriate amount of absolute ethanol to make a concentration of 1% ( g / g) of povidone K-30 absolute ethanol solution, to obtain.
[0062] (3) Mixing and granulation: Weigh the prescribed amount of imatinib, 4 / 7 of the total prescribed amount of microcrystalline cellulose and 1 / 2 of the total prescribed amount of crospovidone, and place them in a rapid granulator, mix well, Add the above-mentioned povidone K30 absolute eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com