Method for determining trace imatinib in blood sample and application thereof to zero phase clinical trial
A technology of tinib meter and medicinal salt, which can be applied to the application of the method in drug zero-phase clinical trial research and the field of drug determination, which can solve problems such as great difference and difficulty in measuring blood drug concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
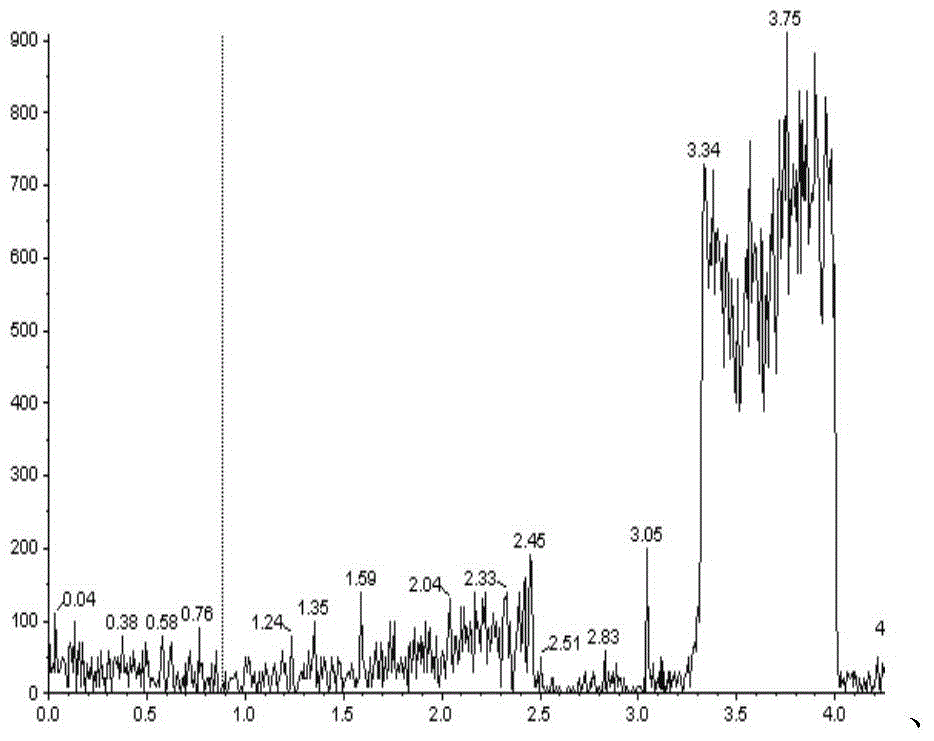
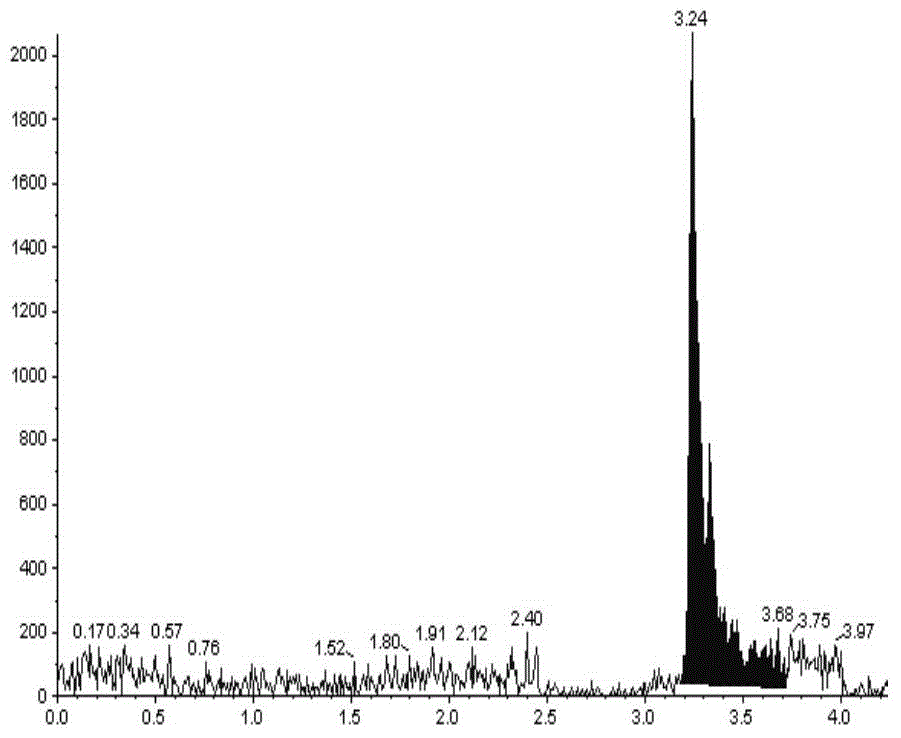
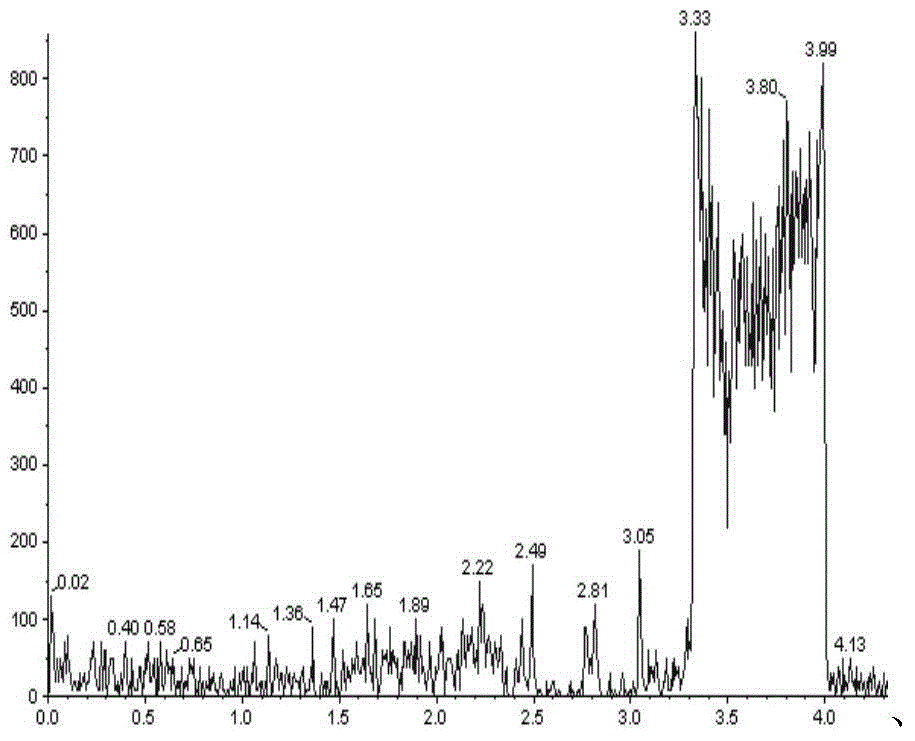
[0086] The present invention can be further described by the following examples, however, the scope of the present invention is not limited to the following examples. Those skilled in the art can understand that various changes and modifications can be made in the present invention without departing from the spirit and scope of the present invention. The present invention provides general and / or specific descriptions of the materials and test methods used in the tests. While many of the materials and methods of manipulation which are employed for the purposes of the invention are well known in the art, the invention has been described here in as much detail as possible.
[0087] In the further description below, if not specified, the imatinib or its pharmaceutically acceptable salt used is imatinib mesylate, and the imatinib mesylate preparation used is a commercially available capsule H20100238. In the following, when the internal standard is used, unless otherwise specifie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com