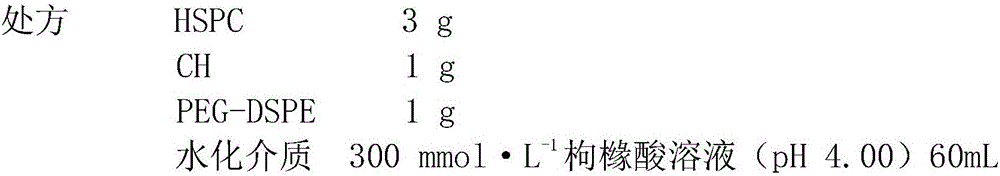Imatinib mesylate liposome preparation and preparation method thereof
A technology of imatinib mesylate and liposome preparations, which is applied in the field of imatinib mesylate preparations, can solve the problems of non-selectivity of tumor cells and harm to normal cells, and achieve enhanced anti-tumor Efficacy, preparation stability, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044] Prepare blank liposomes: Dissolve the prescribed amount of HSPC, CH, and PEG-DSPE (PEG molecular weight is 2000) in 10 mL of absolute ethanol at 55°C; inject into hydration medium preheated to the same temperature, and incubate for 10 minutes to obtain liposomes The primary plastid product: After the initial product was mixed with 200W ultrasonic for 2min, 400W ultrasonically dispersed for 4min (working for 1s, intermittent for 1s), and then passed through 0.8, 0.45, 0.22μm microporous membranes in turn to obtain blank liposomes .
[0045] Prepare drug liposome: get a certain amount of blank liposome, add appropriate amount of sodium phosphate-phosphate buffer (concentration is 100mmol L -1 ) to adjust the pH to 7.0, and inject the drug-lipid ratio of 1:10 (w / w) with imatinib mesylate solution. The measured encapsulation efficiency is 98.6%, and the particle size is 124nm.
[0046] At the same time, on the basis of the injections obtained above, the corres...
Embodiment 2
[0049]
[0050] The preparation process of the liposome initial product is the same as "Example 1", the liposome initial product is processed with micro jet (12000psi handles 2 cycles, 14000psi handles 2 cycles), then passes through 0.8, 0.45, 0.22, 0.10 successively , 0.05 μm microporous membrane to obtain blank liposomes. The blank liposome was loaded with drug according to the drug-lipid ratio and drug-loading conditions of "Example 1", and the liposome encapsulation efficiency was determined to be 95.2%, and the particle size was 116nm.
[0051] After adding trehalose in imatinib mesylate liposomes as a lyoprotectant (the ratio of trehalose to phospholipid dry weight is 4), freeze-dry according to conventional techniques to obtain imatinib mesylate Liposome formulations were lyophilized. When reconstituted, water for injection is added to the lyophilized liposome for reconstitution to obtain the imatinib mesylate liposome preparation. The encapsulation efficiency after...
Embodiment 3
[0053] Liposome membrane material is composed of HSPC, CH and mPEG 2000 -DSPE composed of HSPC and mPEG 2000 -The mass ratio of DSPE is 3:1, and the mass ratio of CH to HSPC is 1:0.5, 1:1, 1:1.5, 1:3, 1:5, 1:10, respectively, and the lipids without adding CH are prepared body. According to the method described in "Example 1", imatinib mesylate liposomes were prepared, and the encapsulation efficiency and particle size of the obtained liposomes are shown in Table 1.
[0054] Table 1 Effect of cholesterol content on liposome particle size and encapsulation efficiency
[0055]
[0056] As can be seen from Table 1, the cholesterol content in the prescription has a greater impact on the encapsulation efficiency and particle size of imatinib mesylate liposomes. When the prescription does not contain CH, the encapsulation efficiency of imatinib mesylate liposome is less than 10.0%; as the cholesterol content increases, the encapsulation efficiency increases gradually, when the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com