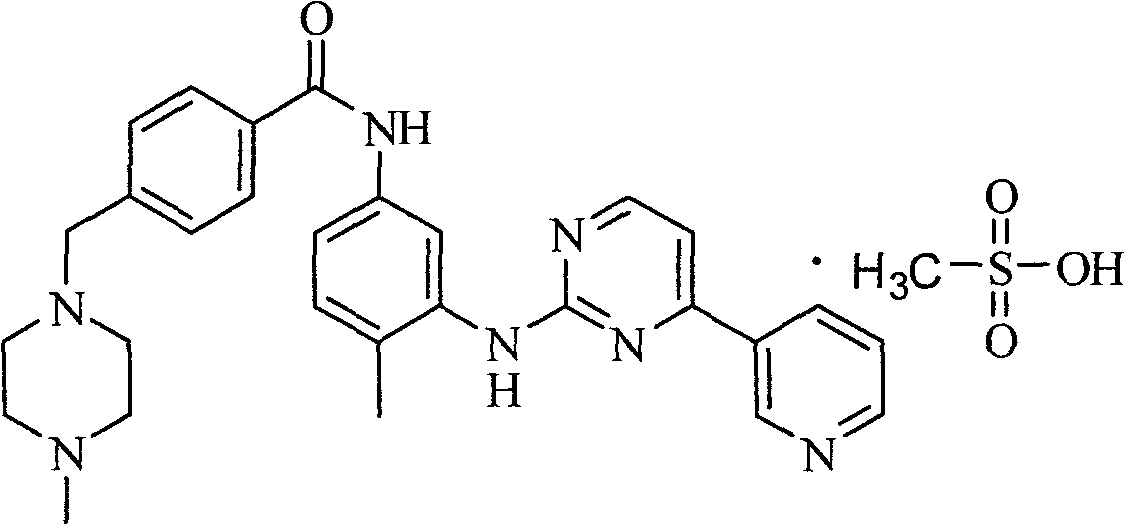
Imatinib mesylate composition and preparation method thereof
A technology of imatinib mesylate and imatinib mesylate, which is applied in the field of imatinib mesylate tablet and its preparation, can solve the problem of incomplete dissolution of tablets, prolonging the stimulation journey, and irritation of the digestive tract and other problems, to achieve the effect of shortening the granulation time, improving safety and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
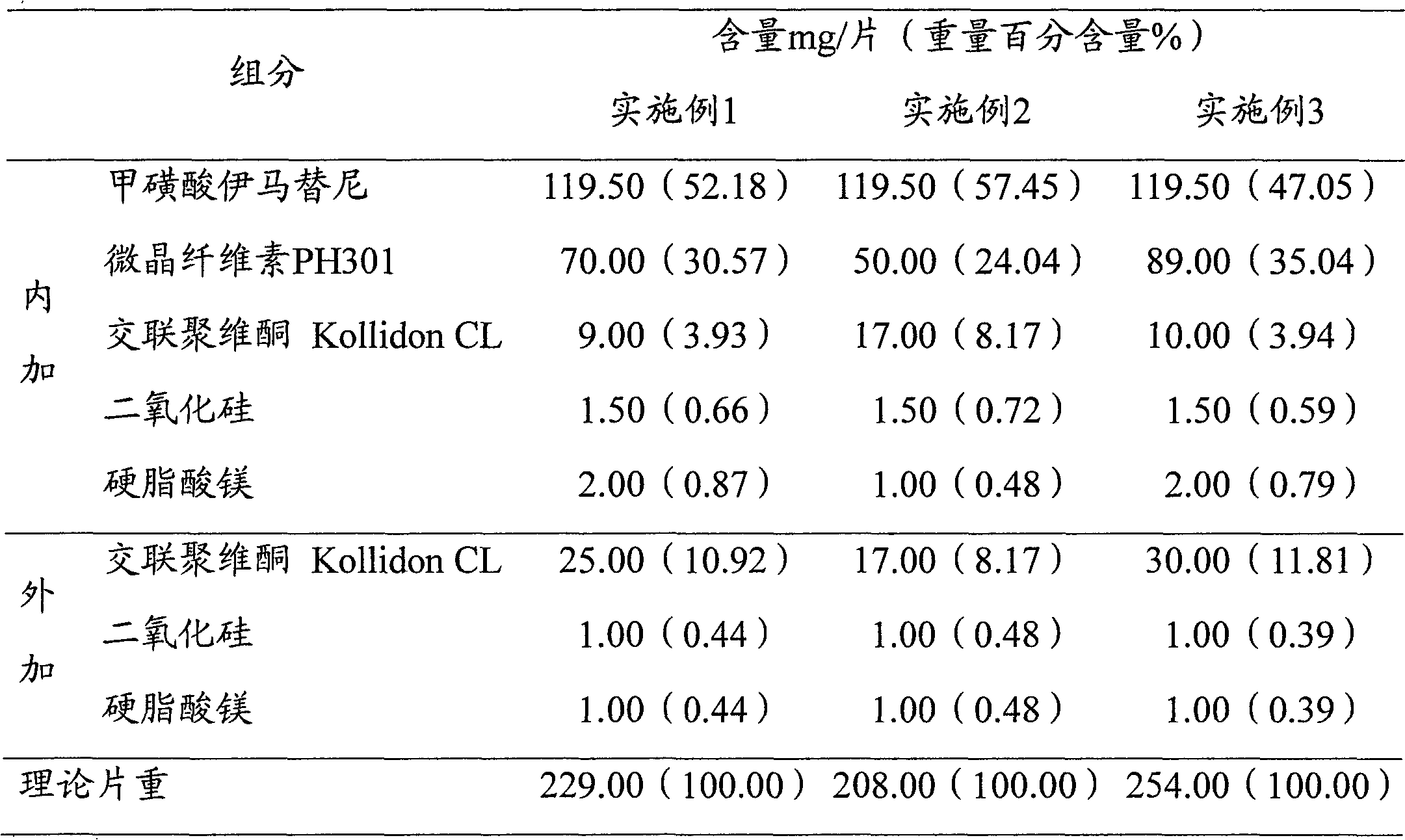
Embodiment 1~3
[0036]
[0037] Preparation Process:
[0038] i) passing imatinib mesylate, microcrystalline cellulose and crospovidone through a 60-mesh sieve for subsequent use;
[0039] ii) Weighing imatinib mesylate, microcrystalline cellulose and the crospovidone added in part are mixed uniformly;
[0040] iii) take by weighing the silicon dioxide and magnesium stearate of adding part, mix homogeneously;
[0041] iv) dry granulator granulation, pressure 5MPa;
[0042] v) According to the proportion of the prescription, add the excipients and mix them evenly;
[0043] vi) Compressed into tablets with a hardness of 6-8kg / cm 2 .
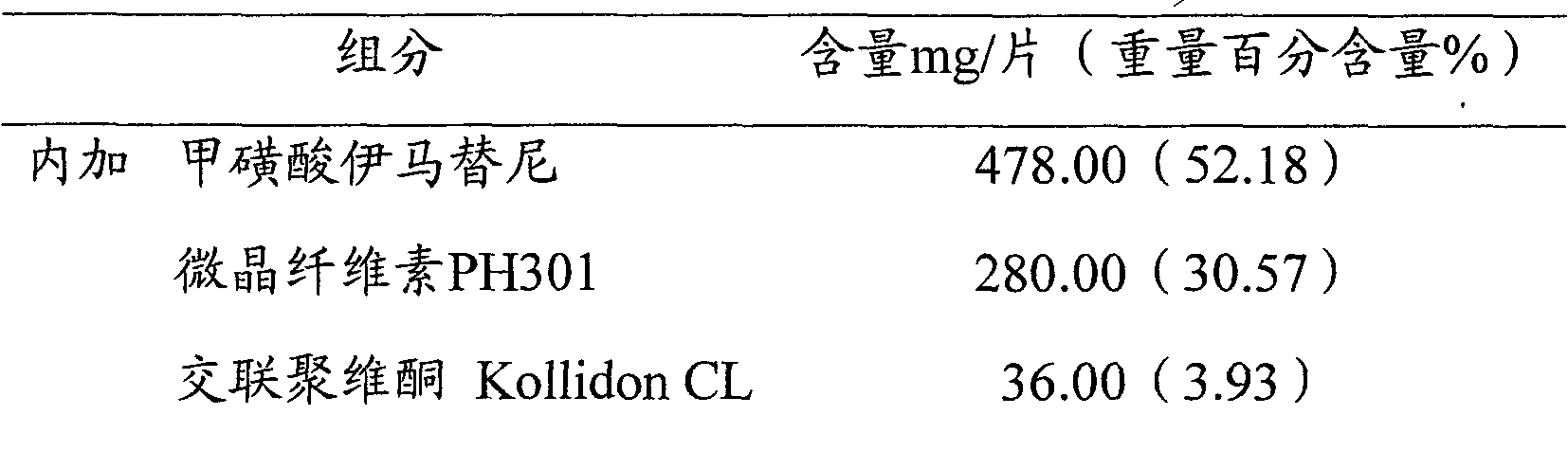
Embodiment 4
[0045] Prescription is the same as embodiment 1
[0046] Preparation Process:
[0047] i) imatinib mesylate is passed through a 200 mesh sieve, and microcrystalline cellulose and crospovidone are passed through a 60 mesh sieve for subsequent use;
[0048] ii) Weighing imatinib mesylate, microcrystalline cellulose and the crospovidone added in part are mixed uniformly;
[0049] iii) take by weighing the silicon dioxide and magnesium stearate of adding part, mix homogeneously;
[0050] iv) dry granulator granulation, pressure 5MPa;
[0051] v) According to the proportion of the prescription, add the excipients and mix them evenly;
[0052] vi) Compressed into tablets with a hardness of 6-8kg / cm 2 .
Embodiment 5
[0054] The tablet prepared in Example 1 was coated with Opadry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com