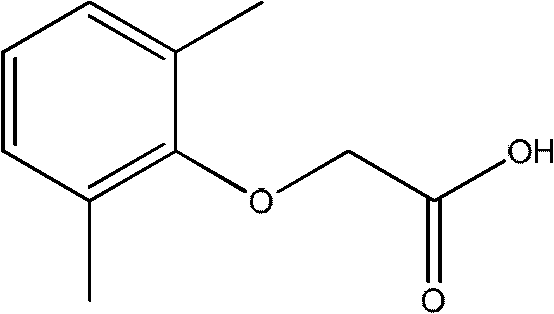
Method for synthesizing 2,6-dimethyl phenoxyacetic acid
A technology of dimethylphenoxyacetic acid and a synthesis method, applied in 2 fields, can solve problems such as being unsuitable for industrial production, expensive raw materials, and large pollution, and achieve the effects of reduced reaction difficulty, easy post-processing, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Add 12.2g (0.1mol) of 2,6-dimethylphenol into a 250ml beaker, heat up to 40°C, add 16g (0.1mol) of 25% NaOH solution, stir and dissolve, filter after fully reacting, and heat up the filtrate Water was removed at 110°C to obtain 12.4 g of solid powder of sodium 2,6-dimethylphenate.
[0030] 2. Take 12.4g (0.086mol) of sodium 2,6-dimethylphenolate and 8.127g (0.086mol) of chloroacetic acid into a 250ml dry three-necked flask, heat up to 150°C under a nitrogen atmosphere, and react at a constant temperature for 1.5 h. After the reaction is completed, wait for the reaction flask to cool down slightly, add 50ml of warm water therein, and shake it. When the solution is cooled to room temperature, the solid crude product can be obtained by filtration. The crude product was recrystallized from ethanol-water to obtain 12.6 g of pure 2,6-dimethylphenoxyacetic acid with a molar yield of 70%.
Embodiment 2
[0032] 1. Add 12.2g (0.1mol) of 2,6-dimethylphenol into a 250ml beaker, add 18g (0.135mol) of 30% NaOH solution, stir and dissolve, filter after fully reacting, and heat up the filtrate to 110°C to remove water , to obtain 13.0 g of solid powder of sodium 2,6-dimethylphenoxide.
[0033] 2. Take 13.0g (0.090mol) of 2,6-dimethylphenate sodium and 8.505g (0.090mol) of chloroacetic acid into a 250ml dry three-necked flask, heat up to 130°C under a nitrogen atmosphere, and react at a constant temperature for 1.5 h. After the reaction is completed, wait for the reaction flask to cool down slightly, add 50ml of warm water therein, and shake it. When the solution is cooled to room temperature, the solid crude product can be obtained by filtration. The crude product was recrystallized from ethanol-water to obtain 13.20 g of pure 2,6-dimethylphenoxyacetic acid with a molar yield of 73.2%.
Embodiment 3
[0035] 1. Add 12.2g (0.1mol) of 2,6-dimethylphenol into a 250ml beaker, add 6.8g (0.17mol) of NaOH and 12g of water, stir and dissolve, filter after fully reacting, and heat up the filtrate to 110°C to remove water to obtain 13.0 g of solid powder of sodium 2,6-dimethylphenate.
[0036] 2. Take 13.0g (0.090mol) of sodium 2,6-dimethylphenolate and 8.505g (0.090mol) of chloroacetic acid into a 250ml dry three-necked flask, heat up to 140°C under a nitrogen atmosphere, and react at constant temperature for 2 hours , after the reaction was completed, the reaction bottle was slightly cooled, 50 ml of 30% NaOH solution was added thereto, shaken, and when the solution was cooled to room temperature, the solid crude product could be obtained by filtration. The crude product was recrystallized from ethanol-water to obtain 13.48 g of pure 2,6-dimethylphenoxyacetic acid with a molar yield of 74.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com