Preparation method for enrofloxacin-d5
A technology of deuterated enrofloxacin and -d5, which is applied in the fields of organic chemistry methods, chemical instruments and methods, isotope introduction of heterocyclic compounds, etc., and can solve the problems such as the inability to obtain isotopic abundances.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
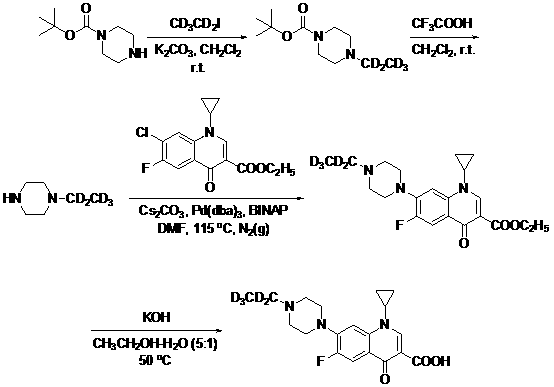
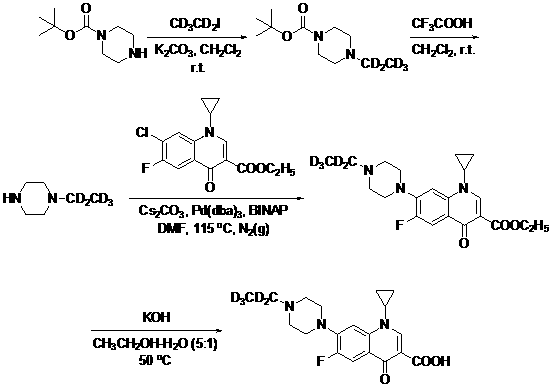
[0052] Example 1: 4-Ethyl-1-Boc-piperazine- d 5 preparation of
[0053] 1-Boc-piperazine (2.42 g, 13 mmol) was mixed with CD 3 cd 2 I (1.47 g, 9 mmol) dissolved in CH 2 Cl 2 (20 mL), add K to the above mixture 2 CO 3 (1.8 g, 13 mmol), stirred at room temperature for 18 h, then added CD 3 cd 2 I (1.47 g, 9 mmol), continue stirring at room temperature for 18 h, after the reaction is complete, add H 2 O (10 mL), split CH 2 Cl 2 layer, water soluble with CH 2 Cl 2 Extract (2 × 10 mL), combine CH 2 Cl 2 layer, washed with saturated NaCl, anhydrous MgSO 4 Drying and concentration under reduced pressure gave 2.72 g yellow oil crude product 4-ethyl-1-Boc-piperazine- d 5 , the yield was 98%, and could be directly used in the next step without purification. ESI-MS(m / z): 220.1 (M+H) +1 , 1 HNMR (400 MHz, CDCl 3 ): δ = 3.43-3.45 (4H, m), 2.38-2.44(4H, m), 1.46 (9H, s).
example 2
[0054] Example 2: 1-Ethyl-piperazine- d 5 preparation of
[0055] 4-Ethyl-1-Boc-piperazine- d 5 (1.50 g, 7 mmol) dissolved in CH 2 Cl 2 (15 mL), to which was added CF 3 COOH (0.62 mL, 8.4 mmol), stirred at room temperature for 10 min, concentrated under reduced pressure to remove CH2 Cl 2 , to which CH 3 OH (10mL), concentrated under reduced pressure again to remove CH 3 OH, 0.79 g yellow oil crude product 1-ethyl-piperazine- d 5 , the yield was 95%, and could be directly used in the next step without further purification. ESI-MS (m / z): 120.1 (M+H) +1 , 1 HNMR (400 MHz, CDCl 3 ): δ =5.1 (1H, br s), 3.51-3.75 (8H, m).
example 3
[0056] Example 3: Ethyl 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-piperazinyl)-3-quinolinecarboxylate- d 5 Preparation (coupling method)
[0057] 1-Ethyl-piperazine- d 5 (0.79 g, 6.7 mmol), ethyl 1-cyclopropyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylate (1.6 g, 5 mmol), Cs 2 CO 3 (3.3 g, 10 mmol), Pd(dba) 3 (0.1 g, 0.1 mmol) and BINAP (0.1 g, 0.15 mmol) were dissolved in DMF (20 mL), N 2 protection, stirred at 115°C for 2 h, after the reaction was completed, concentrated under reduced pressure to remove most of the solvent, and added CH 2 Cl 2 (20 mL), filtered through a sand core, and the filtrate was concentrated under reduced pressure to obtain a brownish-red oily crude product, which was subjected to silica gel column chromatography (CH 3 OH) to obtain 1.8 g of light yellow solid 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-piperazinyl)-3-quinolinecarboxylic acid ethyl ester- d 5 , the yield is 93%. ESI-MS (m / z): 393.1 (M+H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com