Oral capsule for intestinal flora transplantation and preparation method thereof
A technology of intestinal flora and capsules, applied in the field of biomedicine, can solve the problems of changing the ecological balance of intestinal flora, reducing the activity of intestinal flora, and difficult to obtain therapeutic effects, so as to avoid killing and destroying effects and improve success. efficiency, guaranteed abundance and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of oral capsules for intestinal flora transplantation
[0029] The intestinal flora of this example comes from a strictly screened healthy donor, and all operations should be completed within 6 hours after the stool leaves the healthy donor. The screening of healthy donors strictly follows the relevant guidelines of the International Consensus on FMT, the European Consensus on FMT, the Asia-Pacific Consensus on FMT, and the Chinese Children’s Consensus on FMT, and adopts a strict "exclusion method". Screening includes review of medical history, serology to screen for infectious disease, stool examination, sequencing of gut microbiota, and confirmation of absence of gastrointestinal disorders and other neurodevelopmental problems. Serological tests ruled out hepatitis A, B, C, and E infection, human immunodeficiency virus type I / II infection, T-cell tuberculosis infection, TORCH virus infection, and syphilis infection, and liver and kidney function...
Embodiment 2
[0036] Example 2: Comparative test on the protective effect of different glycerol concentrations on the activity of flora in oral capsules
[0037] In order to protect the intestinal flora in the flora transplantation capsule from freezing damage, the present invention adds a non-toxic and edible cryopreservation agent when making the capsule, although most non-permeable macromolecular cryoprotectants are edible materials , but the medium and small capsule matrix of the present invention--sodium alginate solution is a viscous solution, and the non-permeable macromolecular cryoprotectant is difficult to dissolve therein. Therefore, the present invention selects food-grade glycerin as the small capsule cryoprotectant.
[0038] The present invention also examines the protective effect of different concentrations of glycerin on live bacteria in small capsules: the cryoprotectant can protect bacteria or cells from freezing damage during cryopreservation. Cryoprotectants are divided...
Embodiment 3
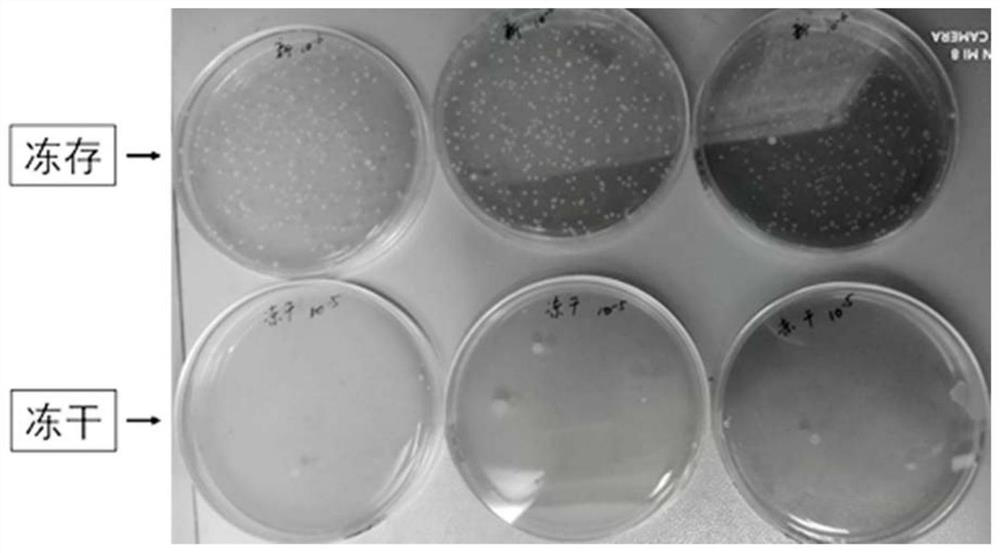
[0049] Embodiment 3: The oral capsule prepared by the present invention adopts the comparative test of the number of viable bacteria and the abundance of flora preserved by freezing and freeze-drying
[0050] (1) The preparation of frozen Enterobacteria small capsules is the same as in Example 1; some small capsules are directly frozen in a -80°C refrigerator, and the other small capsules are processed using a Biocool vacuum freeze dryer (Pilot 2-4H) Freeze drying;
[0051] (2) Take the same amount of frozen (-80°C for one week) and freeze-dried (freeze-dried) small capsules of Enterobacteriaceae, add 10 times the volume of 1% sodium citrate solution, shake and incubate for 30 minutes, and the small capsules are completely dissolved Afterwards, serial dilutions were performed with saline;
[0052] (3) Take 100 μl of frozen samples 10 -6 , freeze-dried samples to take 100μl 10 -6 The diluted bacterial solution was spread on the improved GAM plate, and the plate was placed in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com