Method for preparing deuterated internal standard substances of tetracyclines and epimers thereof
A technology of epimers and tetracyclines, which is applied in the field of preparation of deuterated stable isotope internal standard reagents, can solve the problems that restrict the application of tetracycline stable isotope internal standard reagents, complicated operation process, long technical route, etc. The effect of preparation risk, short technical route, and guaranteed abundance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
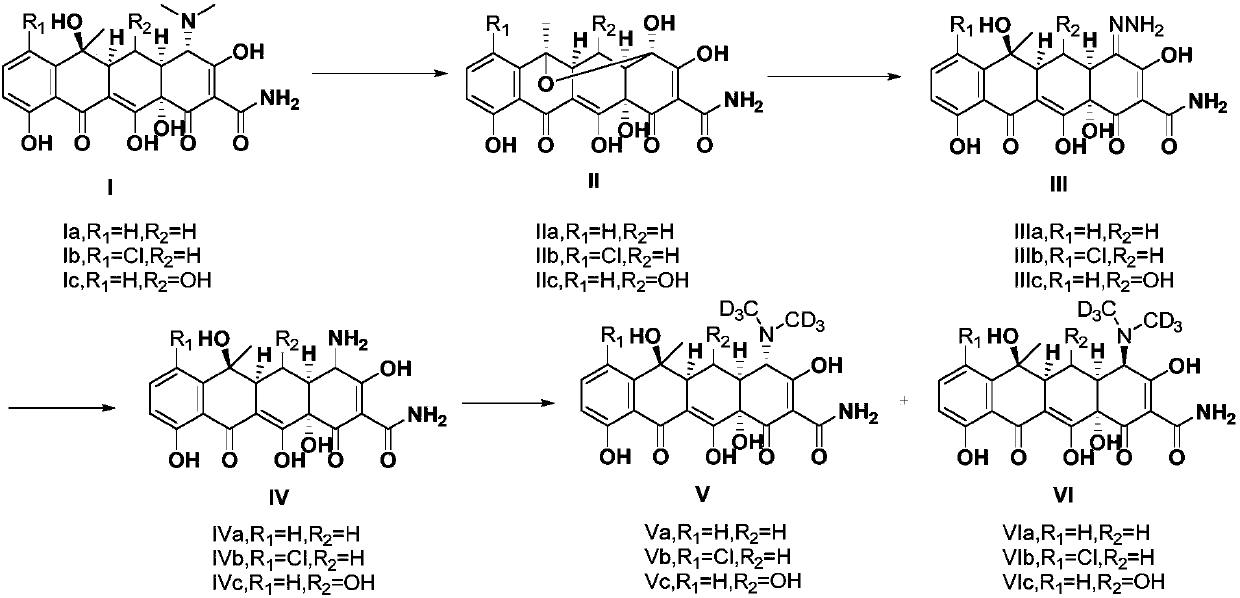
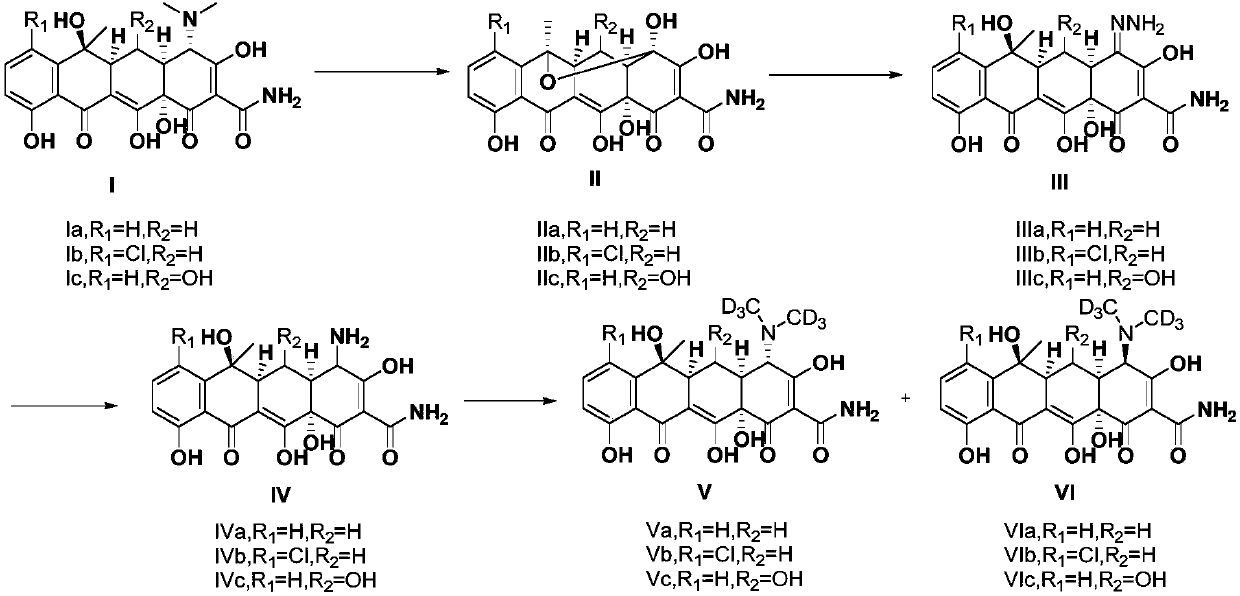
[0026] (1R,4aS,11R,11aS,12aR)-1,2,4a,5,7-pentahydroxy-11-methyl-4,6-dioxomethylene-1,4,4a,6,11 , Preparation of 11a, 12, 12a-octahydro-1,11-epoxytetracene-3-carboxamide (IIa):
[0027] Dissolve 4.6 grams of tetracycline (Ia) in 250 milliliters of aqueous solution containing 1 milliliter of concentrated hydrochloric acid, and add 3.5 grams of N-chlorosuccinimide in batches at 25°C, after the addition is complete, stir and react at 25°C 30 min; filter with suction, wash the filter cake with water, dissolve the filter cake in 300 ml of methyl tert-butyl ether, and wash 4 times with 50 ml of water. After the solvent was spin-dried under reduced pressure, 3.2 g of the product as a light yellow solid was directly used in the next reaction with a yield of 74.4%. LCMS(ESI+): m / z=416[M+H] + .
Embodiment 2
[0029] (4aS,5aS,6S,12aS,E)-4-hydrazino-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxomethylene-1,4, Preparation of 4a, 5, 5a, 6, 11, 12a-octahydro-2-carboxamide (IIIa):
[0030] 3.2g (1R, 4aS, 11R, 11aS, 12aR)-1,2,4a,5,7-pentahydroxy-11-methyl-4,6-dioxomethylene-1,4,4a, 6,11,11a,12,12a-octahydro-1,11-epoxytetracene-3-carboxamide (IIa) is dissolved in 25 ml of 95% ethanol solution, 0.46 A solution of 80% hydrazine hydrate in 25 ml ethanol was slowly added dropwise to the reaction mixture. After dropping, keep warm for 18 hours. After spin-drying the solvent, 3.3 g of a reddish-brown solid was directly used in the next reaction. Yield 99.7%. LCMS(ESI+): m / z=430[M+H] + .
Embodiment 3
[0032] (4aS,5aS,6S,12aS)-4-Amino-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxomethylene-1,4,4a,5 , the preparation of 5a,6,11,12a-octahydro-2-carboxamide (IVa):
[0033] 3.3 grams of (4aS, 5aS, 6S, 12aS, E)-4-hydrazino-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxomethylene-1 , 4,4a,5,5a,6,11,12a-octahydro-2-carboxamide (IIIa) was dissolved in a mixed solution of 130 ml of acetone and 100% of water, and 6.6 g of it was added in batches at 25°C Sodium dithionite, control the temperature at about 30°C, after the addition is complete, stir and react at 25°C for 30min. After the reaction, add 3 grams of activated carbon, stir for 10 minutes, then add diatomaceous earth for suction filtration and wash with a 1 / 1 mixed solution of water and acetone. Combine the filtrates, spin dry the acetone and adjust the pH to 4-5, wash the aqueous layer with ethyl acetate, extract with n-butanol, dry over anhydrous sodium sulfate, and then suction filter and spin dry to obtain 1.3 g of crude ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com