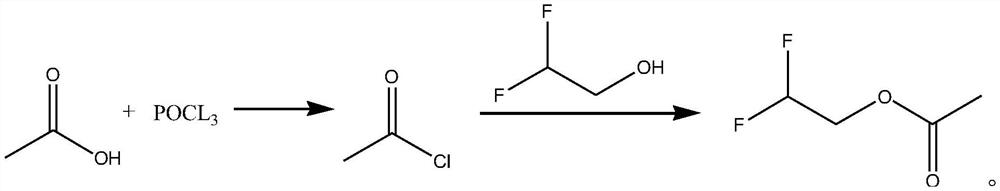
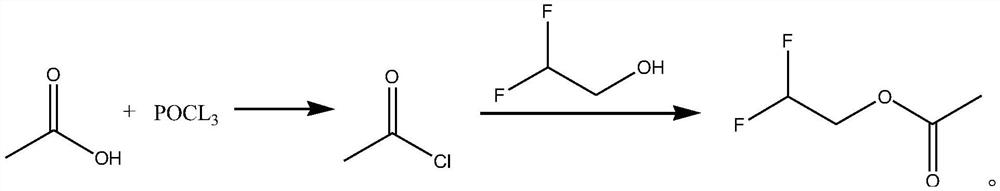
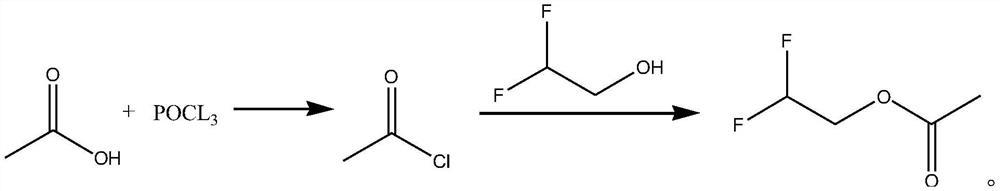
Synthetic method of difluoroethanol acetate
A technique for the synthesis of difluoroethanol acetate, which is applied in chemical instruments and methods, preparation of carboxylic acid salts, preparation of carboxylic acid halides, etc., can solve the problem of high storage conditions of acetyl chloride, affecting product yield and purity, Easy to decompose and other problems, to achieve the effect of reducing the difficulty of the reaction, improving the yield and purity, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthetic method of difluoroethanol acetate, its synthetic steps are as follows:
[0023] Take 18g (0.3mol) of glacial acetic acid and cool it down to 0°C, and slowly add 15.3g (0.1mol) of phosphorus oxychloride dropwise. After the reaction, let stand to separate layers, take the upper layer, and obtain the crude product of acetyl chloride, which can be directly subjected to the acylation reaction without post-treatment and drying;
[0024] Dissolve the obtained acetyl chloride in 30mL of dichloromethane, lower the temperature to 0°C again, slowly add 20g of triethylamine dropwise, maintain 0°C and slowly add 24.6g (0.3mol) of difluoroethanol dropwise (the duration of the dropwise addition is 1h), dropwise After completion, react for 1.5h, then slowly add 81g of triethylamine dropwise (the total amount of triethylamine added twice is 101g, i.e. 1mol), after the addition of triethylamine is completed, react for 1h, heat up to 10°C and continue the reaction to Acetyl...
Embodiment 2
[0027] The synthetic method of difluoroethanol acetate, its synthetic steps are as follows:
[0028] Take 18.6g (0.31mol) of glacial acetic acid and cool it down to 0°C, and slowly add 15.3g (0.1mol) of phosphorus oxychloride dropwise. After the acetylation reaction, let stand to separate layers, take the upper layer, and obtain the crude product of acetyl chloride, which can be directly carried out the acylation reaction without post-treatment and drying;
[0029] Dissolve the obtained acetyl chloride in 35mL of dichloromethane, lower the temperature to 5°C again, slowly add 20g of pyridine dropwise, and slowly add 23.78g (0.29mol) of difluoroethanol dropwise at 5°C (the duration of dropping is 40min). , react for 1 hour, then slowly add 67.01g of pyridine dropwise (the total amount of pyridine added twice is 87.01g, that is, 1.1mol). 50 mL of water was added for washing, and the phases were separated. The obtained organic phase was dried over anhydrous magnesium sulfate and...
Embodiment 3
[0031] The synthetic method of difluoroethanol acetate, its synthetic steps are as follows:
[0032] Take 18.3g (0.305mol) of glacial acetic acid and cool it down to 1°C, and slowly add 15.3g (0.1mol) of phosphorus oxychloride dropwise. After the acetylation reaction, let stand to separate layers, take the upper layer, and obtain the crude product of acetyl chloride, which can be directly carried out the acylation reaction without post-treatment and drying;
[0033] Dissolve the obtained acetyl chloride in 28mL of dichloromethane, lower the temperature to 1°C again, slowly add 15g of triethylamine dropwise, maintain 1°C and slowly add 24.19g (0.295mol) of difluoroethanol dropwise (the duration of dropping is 50min), dropwise After completion, react for 1.8h, then slowly add 75.9g of triethylamine dropwise (the total amount of triethylamine added twice is 90.9g, i.e. 0.9mol), after the addition of triethylamine is completed, react for 1.5h, and heat up to 11 Continue the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com