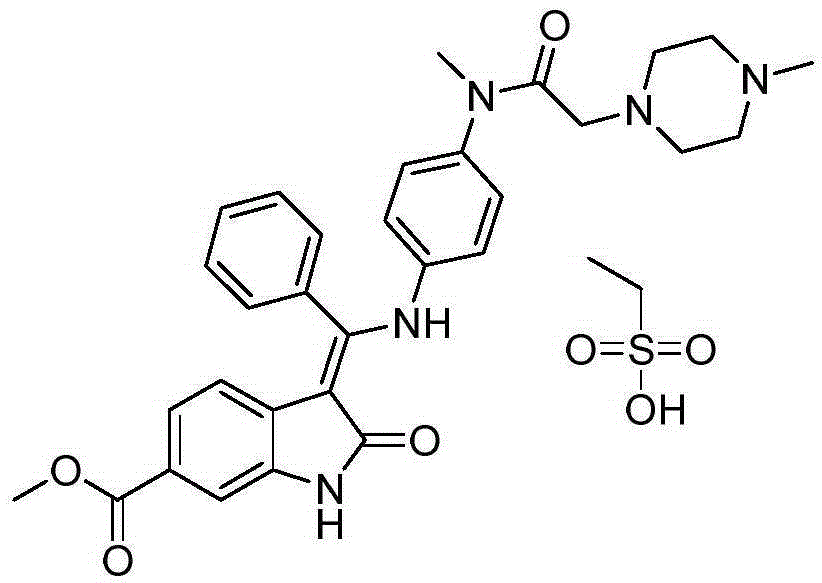
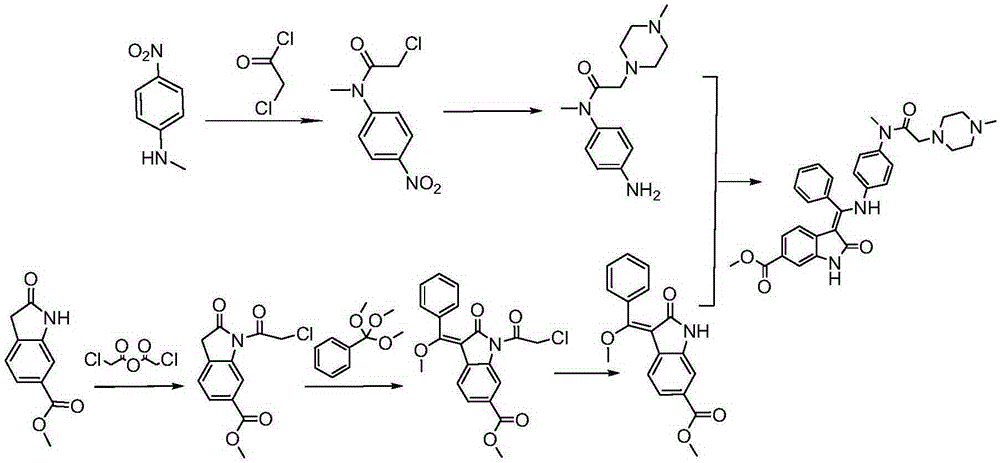
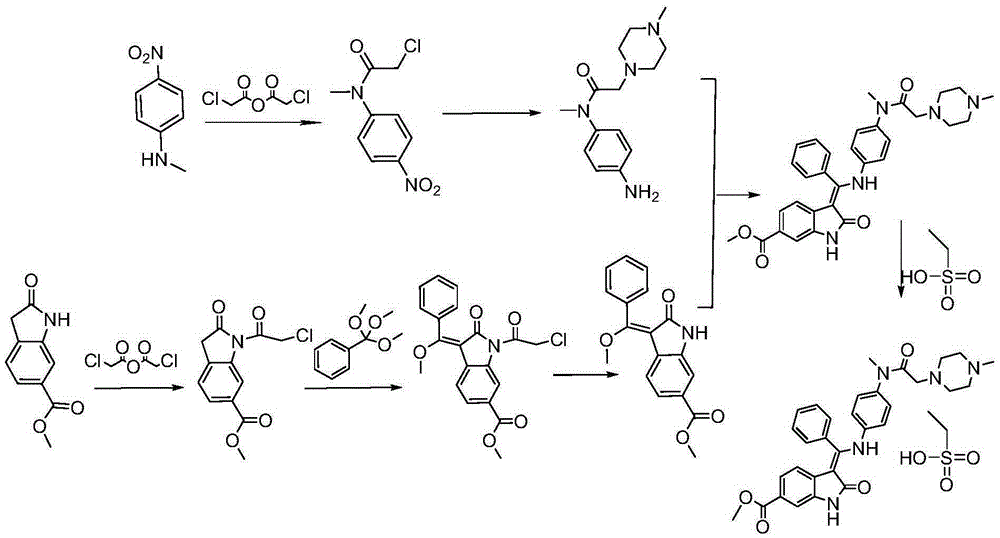
Preparation method of crystalline nintedanib esylate
A technology of nintedanib ethanesulfonate and crystalline form, applied in the field of chemical drug synthesis, can solve the problems of low total yield, long synthesis steps, unsuitable for industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 Preparation of crystalline nintedanib ethanesulfonate
[0039] (1) Synthesis of 1-(chloroacetyl chloride)-2-oxoindoline-6-formic acid methyl ester (formula (C))
[0040] In a 3000mL four-neck round bottom flask, install mechanical stirring, reflux condenser, thermometer, and add 1500mL toluene, 240g 6-methoxycarbonyl-2-oxoindoline, 272g chlorine dioxide to the round bottom flask under nitrogen protection. Acetic anhydride, start stirring, heat up to 80-90°C, keep warm and stir for 6 hours, turn off heating and naturally drop to 80°C, add 600ml cyclohexane, continue stirring to cool down, drop to room temperature, solids precipitate, suction filter, filter cake Wash with ice ethanol and dry in vacuo to obtain 233.6g. Yield: 78%.
[0041] (2) Synthesis of (1-(chloroacetyl)-3-[methoxy(phenyl)methylene]-2-oxoindoline-6-formic acid methyl ester (formula (D))
[0042] In a 5000mL three-necked flask, 195g of methyl 1-(chloroacetyl chloride)-2-oxoindoline-6-carb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com