Preparation method of rosuvastatin intermediate and intermediate compound
A technology of rosuvastatin and compounds, applied in the field of drug synthesis, can solve the problems of high toxicity, etc., and achieve the effect of avoiding pollution, reducing the difficulty of reaction, and fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] (1): Preparation of isobutyrylacetonitrile
[0063]
[0064] 60% NaH (mass percentage concentration, 8.93g, 0.22mol) was added to THF (150mL), heated to 60°C, and a mixture of ethyl isobutyrate (21.6g, 0.186mol) and acetonitrile (9.14g, 0.22mol) was added dropwise , dripped within 1h, continued to react for 6h, GC showed that the reaction of raw materials was almost complete, stopped, evaporated to dryness under reduced pressure, the residue was dissolved in water (300mL), CH 2 Cl 2 Wash (50mL×2), adjust pH3 Wash with solution (50 mL), wash with water (50 mL), and evaporate to dryness under reduced pressure to obtain isobutyrylecetonitrile (19.17 g, yield 88.4%) as a red oil, with a GC purity of 95.2%.
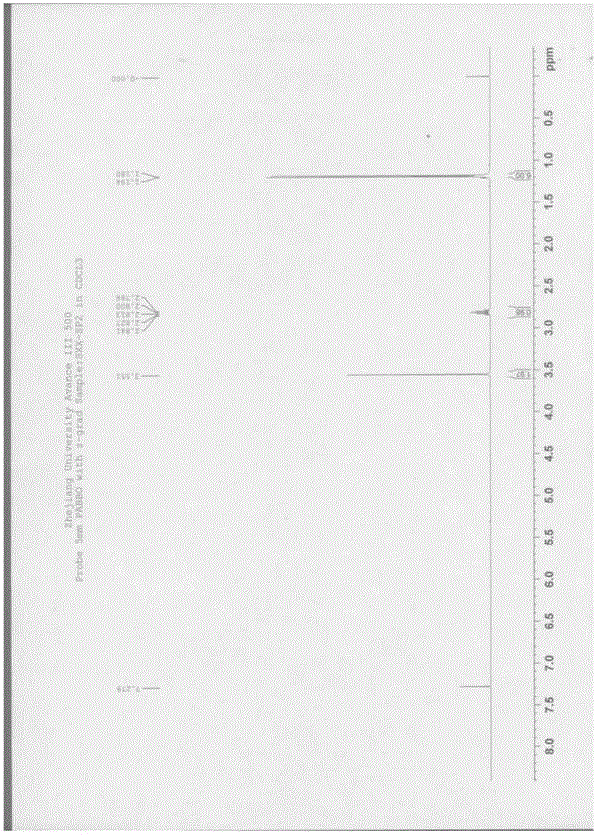
[0065] 1 HNMR (500MHz, CDCl 3 ): 1.18-1.19 (d, 6H); 2.79-2.84 (m, 1H); 3.55 (s, 2H). Such as figure 1 shown.
[0066] (2): Preparation of 4-(4-fluorophenyl)-6-isopropyl-2-thio-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (compound 2)
[0067]
[0068] Mix is...
Embodiment 2
[0084] (1): Preparation of isobutyrylacetonitrile
[0085] Calcium hydride (15.62g, 0.37mol) plus THF (150mL), heated to 60 ° C, dropwise added ethyl isobutyrate (21.6g, 0.186mol) and acetonitrile (11.44g, 0.28mol) mixture, dropwise completed within 1h , continued to react for 6h, GC showed that the reaction of raw materials was almost complete, stopped, evaporated to dryness under reduced pressure, the residue was dissolved in water (300mL), CH 2 Cl 2 Wash (50mL×2), adjust pH3 Wash with solution (50 mL), wash with water (50 mL), and evaporate to dryness under reduced pressure to obtain isobutyrylecetonitrile (19.61 g, yield 89.3%) as a red oil, with a GC purity of 94.0%.
[0086] (2): Preparation of 4-(4-fluorophenyl)-6-isopropyl-2-thio-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (compound 2)
[0087]Mix isobutyrylacetonitrile (1.00g, 9.00mmol), p-fluorobenzaldehyde (1.23g, 9.90mmol), thiourea (1.37g, 18.00mmol), add EtOH (10mL), CuBr (0.01g, 0.09mmol) , Sodium bisulfate (...
Embodiment 3
[0096] (1): Preparation of isobutyrylacetonitrile
[0097] Add THF (150mL) to 27% sodium methoxide methanol solution (mass percentage, 55.80g, 0.28mol), heat to 60°C, add ethyl isobutyrate (21.6g, 0.186mol) and acetonitrile (11.44g, 0.28mol) dropwise ) mixture, dripped within 1h, continued to react for 6h, GC showed that the reaction of raw materials was almost complete, stopped, evaporated to dryness under reduced pressure, the residue was dissolved in water (300mL), CH 2 Cl 2 Wash (50mL×2), adjust pH3 Wash with solution (50 mL), wash with water (50 mL), and evaporate to dryness under reduced pressure to obtain isobutyrylecetonitrile (19.14 g, yield 86.6%) as a red oil, with a GC purity of 93.4%.
[0098] (2): Preparation of 4-(4-fluorophenyl)-6-isopropyl-2-thio-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (compound 2)
[0099] Mix isobutyrylacetonitrile (1.00g, 9.00mmol), p-fluorobenzaldehyde (1.45g, 11.70mmol), thiourea (1.03g, 13.50mmol), add EtOH (10mL), ferrous chloride...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com