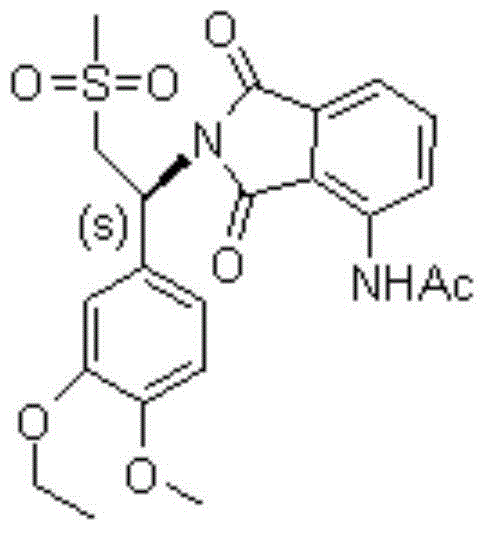
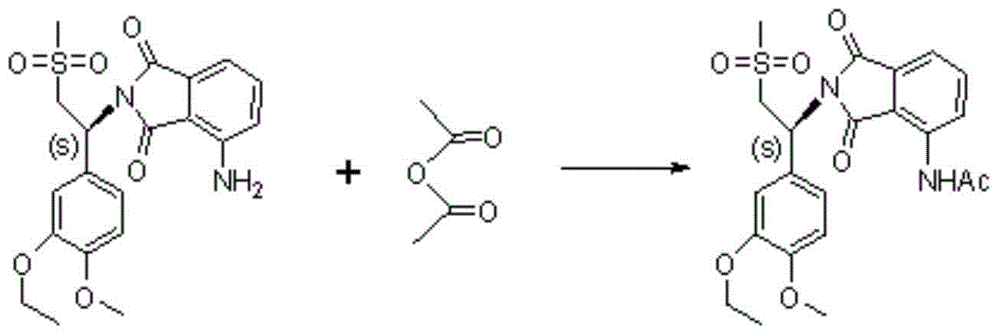
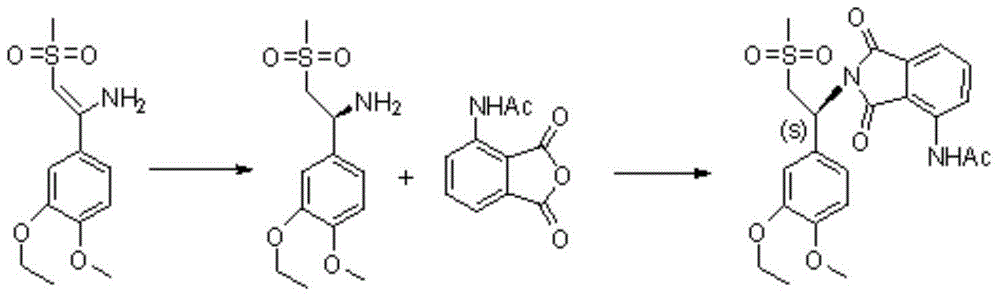
Preparation method of S-type apremilast
A compound and reaction temperature technology, applied in organic chemistry and other fields, can solve the problems of large environmental pollution and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The above-mentioned content of the present invention will be described in further detail below through the embodiment form, but this should not be interpreted as the scope of the above-mentioned theme of the present invention is limited to the following embodiments, all technologies realized based on the above-mentioned content of the present invention belong to this invention the scope of the invention.
[0029] The preparation of embodiment S type apremilast
[0030] (1) Synthesis of compound 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamine L-leucine salt (formula (Ⅲ))
[0031] In a 1000mL four-neck round bottom flask, install mechanical stirring, reflux condenser, and thermometer, add 500ml of methanol to the round bottom flask, start stirring, add 64g of 1-(3-ethoxy-4-methoxyphenyl) -2-(methylsulfonyl)ethylamine, heat up to 70~80℃, heat to reflux to 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamine Dissolve completely, add 22g of N-acetyl-L-leucin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com