Preparation method of tofacitinib citrate
A technology of tofacitinib and citric acid, applied in the field of synthetic drugs, can solve the problems of incomplete reaction, troublesome post-processing, long reaction time and the like, and achieve the effects of less reaction time, short synthesis steps and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
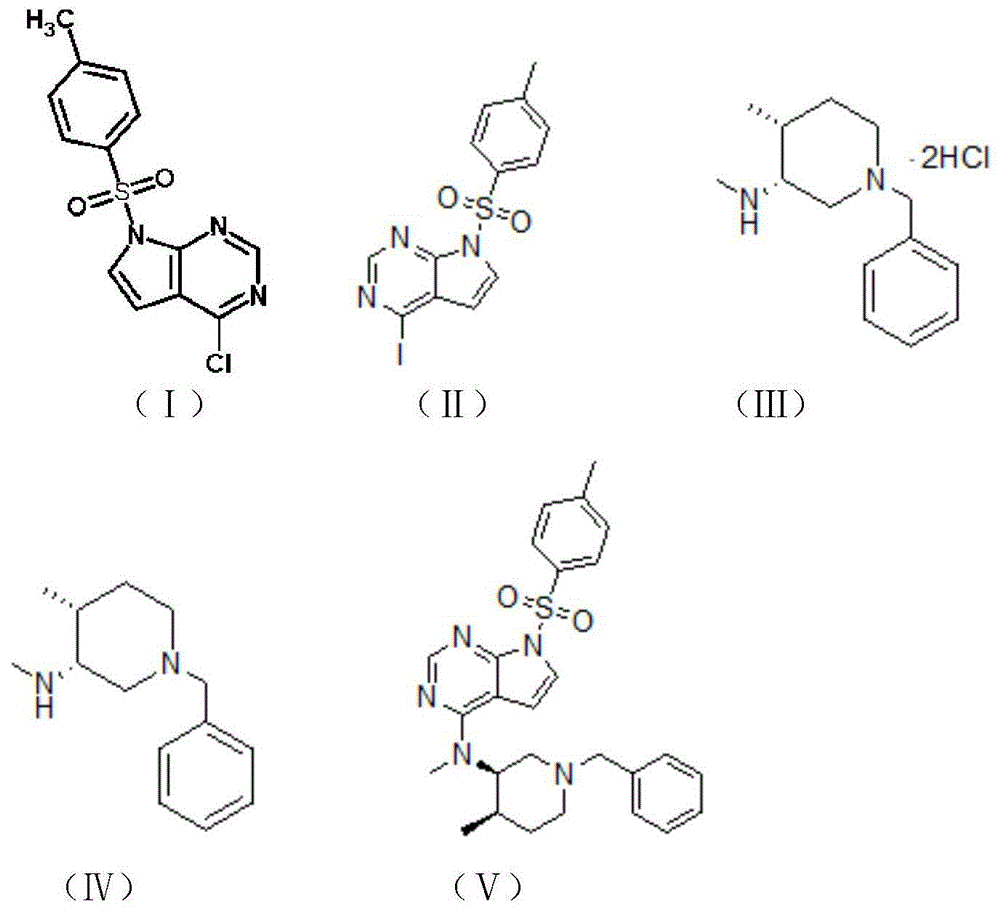
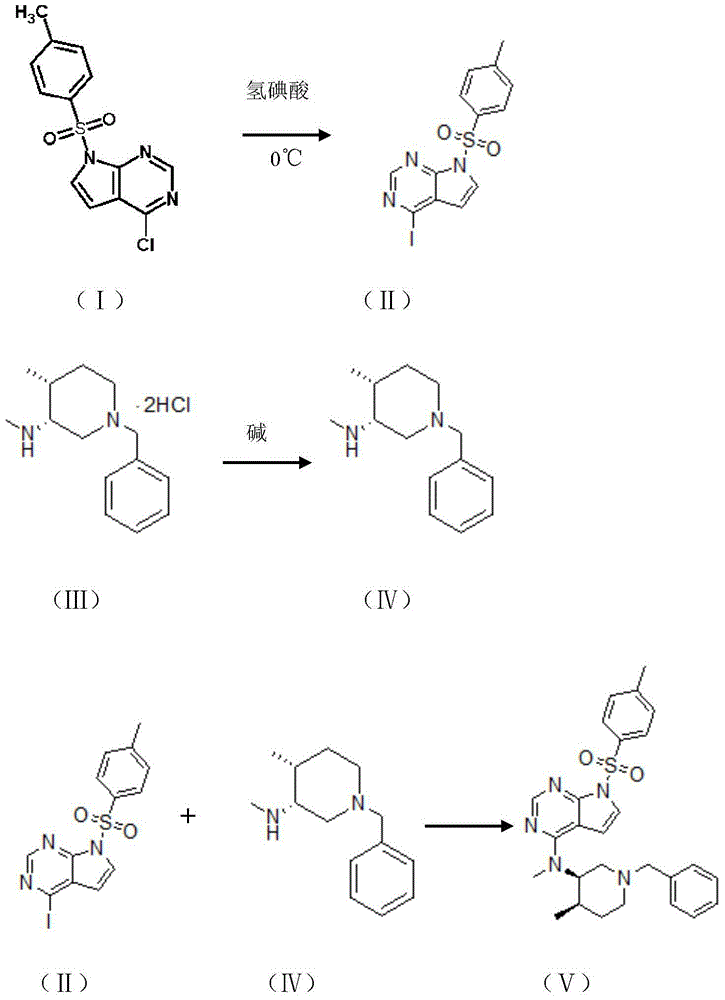
[0028] The preparation method of tofacitinib citrate of the present invention comprises the following steps:
[0029] (1) 8-13mol of compound I is added in the hydroiodic acid of stirring treatment, carries out displacement reaction, stirs, then
[0030] Then raise the temperature to room temperature and stir to obtain compound II;
[0031] (2) Dissolving 1-4mol of compound III in a solvent, adding a base, and performing neutralization reaction to obtain compound IV;
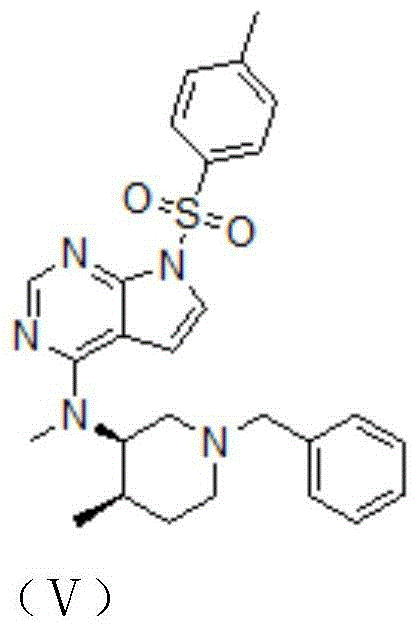
[0032] (3) Add compound II to react compound II and compound IV. During the reaction process, it is necessary to heat up and stir, then cool down, wash and beat under reflux to obtain compound V, which is tofacitinib citrate.
Embodiment 1
[0034] Take 339.5g of compound I whose chemical name is 4-chloro-7-toluenesulfonyl-7H-pyrrole[2,3-D]pyrimidine and add
[0035] In 100ml of 47% hydroiodic acid stirred at 0°C, the stirring time was 1h, and then the temperature was raised to room temperature and stirred for 5h. Then filter, separate and obtain the solid substance, then dissolve the obtained solid with dichloromethane, wash twice with saturated sodium chloride, dry over anhydrous sodium sulfate, and then rotary evaporate to dryness to obtain 439.1g chemically named as 4-iodo-7 -Compound II of tosyl-7H-pyrrolo[2,3-D]pyrimidine.
[0036] In a 1000mL round bottom flask, install a mechanical stirrer, a reflux condenser, and a thermometer, add 600mL of purified water to the round bottom flask, start stirring, and add 29.1g of (3R,4R)-1-benzyl-N,4 -Dimethylpiperidin-3-amine. Compound III of hydrochloride. After compound III is dissolved, slowly add 42.4 g of anhydrous sodium carbonate while stirring to prepare (3R,4R...
Embodiment 2
[0043] Take 246.9g of compound I whose chemical name is 4-chloro-7-toluenesulfonyl-7H-pyrrole[2,3-D]pyrimidine and add it to 100ml of 47% hydroiodic acid stirred at 0°C for 0.5h. Then raise the temperature to room temperature and stir for 4h. Then filter, separate and obtain the solid substance, then dissolve the obtained solid with dichloromethane, wash twice with saturated sodium chloride, dry over anhydrous sodium sulfate, and then rotary evaporate to dryness to obtain 319.4.5g chemical name of 4-iodo- Compound II of 7-tosyl-7H-pyrrolo[2,3-D]pyrimidine.
[0044]In a 1000mL round-bottomed flask, install a mechanical stirrer, a reflux condenser, and a thermometer. Add 600mL of N,N-dimethylformamide to the round-bottomed flask, start stirring, and add 58.2g of (3R,4R)- 1-benzyl-N,4-dimethylpiperidin-3-amine.Compound III of hydrochloride, after compound III is dissolved, slowly add anhydrous sodium bicarbonate 80g while stirring, to prepare chemical name (3R ,4R)-1-benzyl-N,4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com