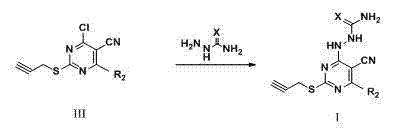
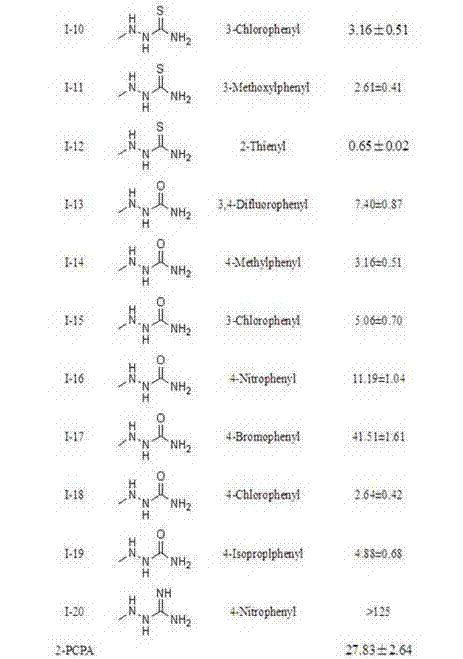
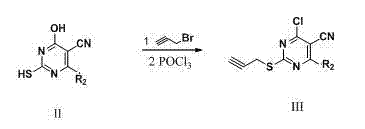
Pyrimidine derivatives containing semicarbazide and terminal alkyne structural units, and preparation methods and applications of pyrimidine derivatives
A technology of pyrimidine derivatives and structural units, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 general formula( II ), R 2 = Derivatives of Phenyl( II-1 ) preparation
[0077] Add ethyl cyanoacetate (2.3g, 20mmol) and sodium hydroxide (1.2g, 30mmol) into the ethanol solution, under reflux conditions, react for a period of time, then add thiourea (2.3g, 30mmol) and benzaldehyde (3.2 g, 30mmol) was added into the reaction system, stirred and reacted, followed by TLC detection. After the reaction, filter with suction and recrystallize to obtain the pure product. Yield 92%, yellow solid.
Embodiment
[0078] Example 2 general formula( III ), R 1 = Cl, R 2 = Derivatives of Phenyl( III-1 ) preparation
[0079] Add propyne bromide (3.6g, 30mmol) dropwise into the solution of II-1 (2.7g, 10mmol) in 1,4-dioxane, and heat and stir at 60°C for reaction. Monitor the reaction process with TLC until the reaction is complete; without separation, directly dropwise phosphorus oxychloride (4.6g, 30mmol) in the reaction system, after the reaction is completed, it is poured into ice water, stirred, and solids are separated out, pumped Filtration to obtain a solid, the pure compound III-1 solid was obtained by column chromatography. Yield 64%, pale yellow solid. Melting point: 131-132 o c. 1 H NMR (400 MHz, CDCl 3 , δ, ppm) δ 8.20 – 8.07 (m, 2H, Ar-H), 7.70 – 7.52 (m, 3H, Ar-H), 4.01 (d, J = 2.6 Hz, 2H, -CH 2 -), 2.28 (t, J = 2.6 Hz, 1H, ≡C-H). 13 C NMR (100 MHz, CDCl 3 C 14 h 9 ClN 3 S, [M+H] + m / z: 286.0206, found: 286.0202.
[0080] Example 3 general formula( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com