Nasal administration of benzodiazepine hypnotics
a benzodiazepine and hypnotics technology, applied in the direction of biocide, heterocyclic compound active ingredients, aerosol delivery, etc., can solve the problems of inefficient many drugs exhibit a number of drawbacks, and many are often inefficiently and variably absorbed from current dosage forms, etc., to facilitate administration, improve duration, efficient and precisely control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In two comparative studies separated in time by over one week; four healthy male, 2-3 year old beagle dogs received oral and nasal doses of triazolam. They were fasted overnight before each study and food was withheld until the end of the experiment. They were restrained in a dog sling during the studies while blood (3 ml) was withdrawn from each dog through a cannula inserted into the cephalic vein.
Oral Administration Studies: Two 0.5 mg triazolam tablets were given to each dog with 50 ml of water. Blood samples were taken from the cephalic vein at 0 min before administration and 15, 30, 45, 60, 120, 180, 240, 300, 360 and 420 min after administration. The plasma samples were stored frozen until gas chromotographic assay for triazolam.
Nasal Administration Studies: Thirty milligrams of triazolam powder was dissolved in 5 ml of PEG 400 warmed at 55.degree.-60.degree. C. After the solution was cooled to room temperature, an equal volume of 1% methocel J5MS (Dow Chemical Company, Midla...
example 2
The efficacy of oral versus nasal administration of midazolam was examined using the methodology of Example 1, but modified to allow at least three weeks between studies.
Oral Administration Studies: Five mg equivalent of midazolam free base solution was given to each of four dogs with 50 ml of water. Blood samples were taken from the cephalic vein at 0 min before administration and 15, 30, 45, 60, 90, 120, 180, 240, 300, 360 min after administration. The plasma samples were stored frozen until GC assay for midazolam.
Nasal Administration Studies: 55.6 milligrams of midazolam HCl powder was dissolved in 4 ml of distilled water. One ml of 7.5% methocel J5MS (Dow Chemical Company, Midland, Mich.) solution was mixed with the midazolam solution. Air bubbles generated during mixing were removed by centrifugation. The pH of the solution was 3.62.
Midazolam solution was sprayed, using a meter dose inhaler into both nostrils of the dogs. The dose given to each dog was 7.85, 7.89, 7.22, 5.61 mg...
example 3
The efficacy of oral versus nasal administration was examined using the methodology of Example 2.
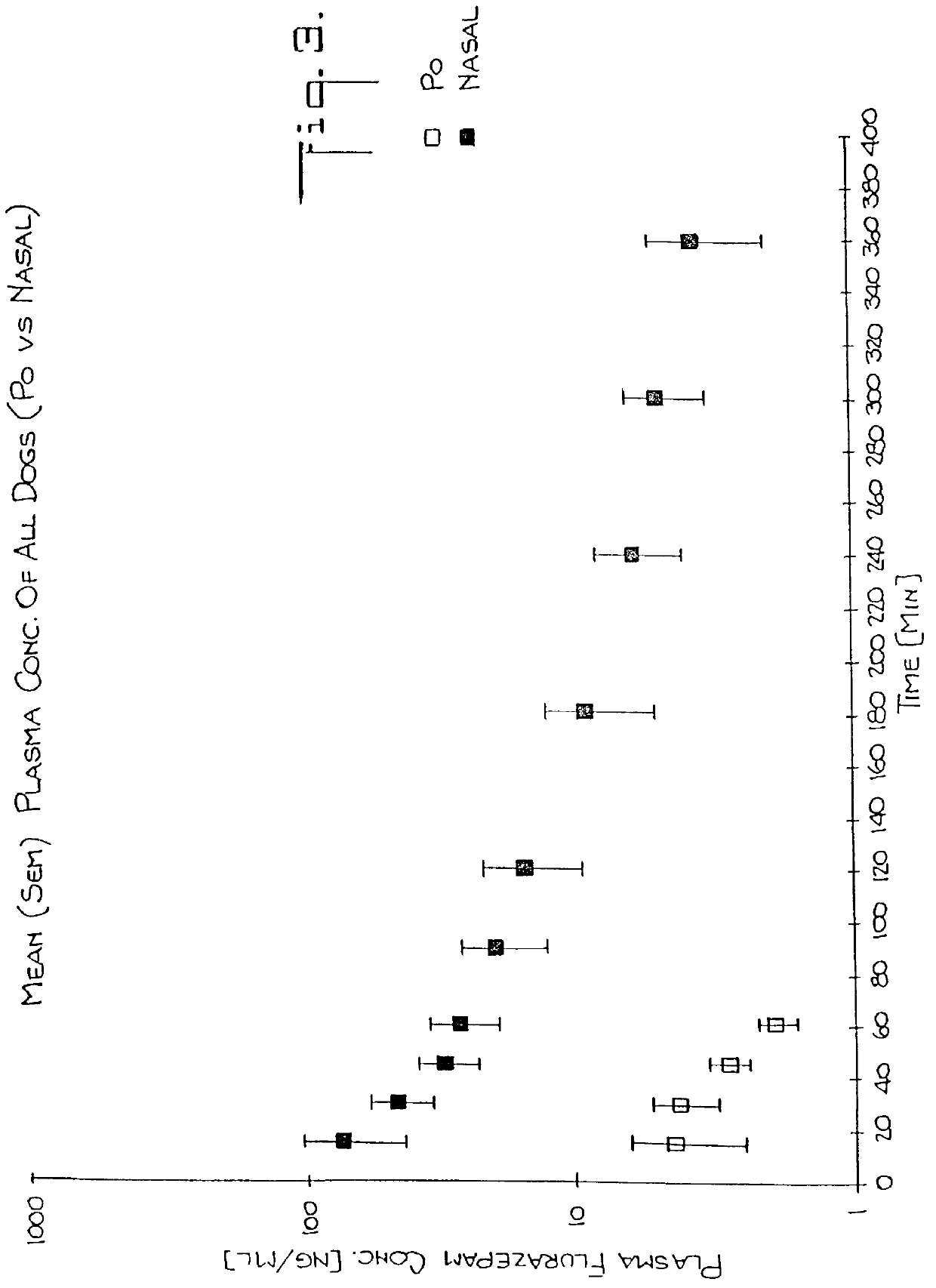
Oral Administration Studies: Fifteen mg of flurazepam HCl solution was given to each dog with 50 ml of water. Blood samples were taken from the cephalic vein at 0 min before administration and at 15, 30, 45, 60, 120, 180, 240, 300, 360 min after administration. The plasma samples were stored frozen until GC assay for flurazepam.
Nasal Administration Studies: One hundred and twenty milligrams of flurazepam HCl powder was dissolved in 4 ml of distilled water. One ml of 7.5% methocel J5MS (Dow Chemical Company, Midland, Mich.) solution was mixed with the flurazepam solution. Air bubbles generated during mixing were eliminated by centrifugation. The pH of the solution was 1.82.
Flurazepam solution was sprayed into both nostrils of the dogs. The dose given to the dogs was 14.5, 12.4, 12.1 and 12.5 mg as flurazepam HCl. Blood sampling times were same as in the oral study. The plasma samples were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com