Preparation method for insulin aspart through recombinant expression by using yeast
A technology of insulin aspart and yeast, which is applied in the field of recombinant protein preparation under biotechnology, can solve the problems of high enzyme dosage and low yield, and achieve the effects of high enzyme cutting efficiency, high coupling rate and low miscutting rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0038] Example 1. Upstream construction and screening of recombinant insulin aspart
[0039](1) Design its cDNA sequence based on the amino acid sequence of recombinant human proinsulin proaspart, and design two stop codon sequences TGA and TAA behind the sequence, and design XhoI (CTCGAG) and NtoI ( GCGGCCGC) restriction enzyme site. Entrust Dalian TAKARA Engineering Co., Ltd. to carry out the whole gene synthesis.
[0040] (2) Insert the Pro-AspcDNA synthesized by the whole gene above into the PMD18-T-Pro-Asp plasmid. PMD18-T-Pro-Asp and pPICZαA were digested with restriction enzymes respectively, and two target fragments (Pro-Asp and pPICZαA large fragments) were recovered, linked by T4 and transformed into P. PastorisX-33 host bacteria to obtain Engineering bacteria (pPICZαA-Pro-Asp / X-33).
[0041] (3) By screening highly expressed engineering bacteria and optimizing the fermentation process, recombinant proinsulin aspart was obtained.
[0042] SEQ ID NO: 1 (InsulinA...
example 2
[0051] Example 2. Preparation of insulin aspart
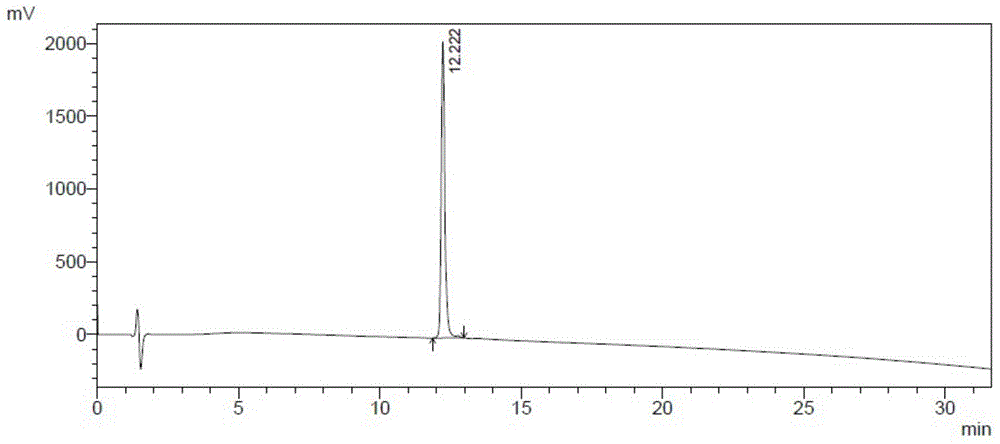
[0052] (1) Purification to obtain proinsulin aspart and enzymatic digestion to obtain missing B30-insulin aspart: 5L of Pichia pastoris fermentation broth, centrifuged, and the supernatant was subjected to copper ion chelation chromatography and SP cation exchange chromatography to obtain aspart Proinsulin; then add 20mM Zn ion to precipitate proinsulin aspart, centrifuge, dissolve the precipitate with 100ml50mMTris, the protein content is 1g, adjust the pH to 8.8, and add lysyl endopeptidase ( Lys-C) 0.2mg, digestion at 30°C with stirring. After 16 hours, samples were taken for RP-HPLC analysis, and the enzyme digestion efficiency reached 90%, and the pH was adjusted to 5.5 for isoelectric point precipitation.
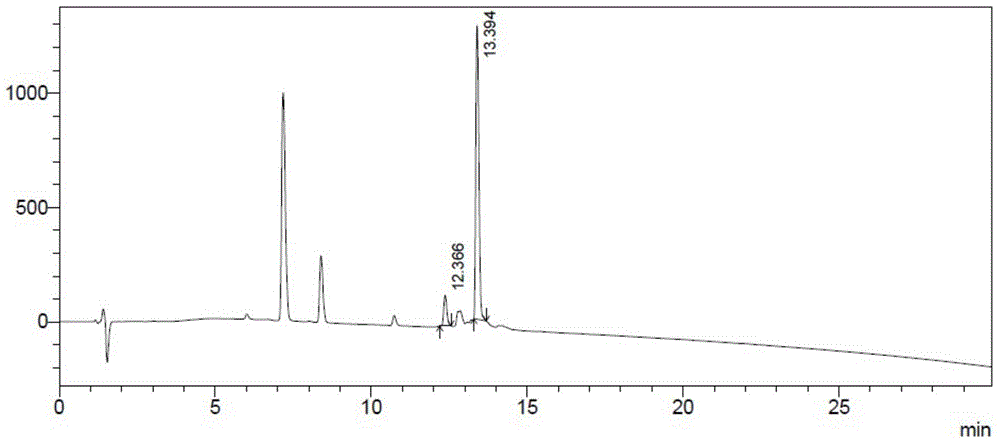
[0053] (2) Coupling of desB30-insulin aspart to obtain insulin aspart ester: take the isoelectric point precipitate desB30-insulin aspart and centrifuge, dissolve with 5ml of 10% glacial acetic acid, the protein con...
example 3
[0057] Example 3. Insulin aspart preparation process optimization
[0058] (1) Purification to obtain proinsulin aspart and enzymatic digestion to obtain DesB30-insulin aspart: Pichia pastoris fermentation broth, centrifugation, supernatant per copper ion chelation chromatography and SP cation exchange chromatography for proaspart; Then add Zn to precipitate proinsulin aspart, centrifuge, dissolve the precipitate with 300ml50mM Tris, the protein content is 2.5g, adjust the pH to 9.0, and add lysyl endopeptidase (Lys-C ) 0.25mg, digested with stirring at 37°C. After 16 hours, samples were taken for RP-HPLC analysis, and the enzyme digestion efficiency reached 95%, and the pH was adjusted to 5.0 for isoelectric point precipitation.
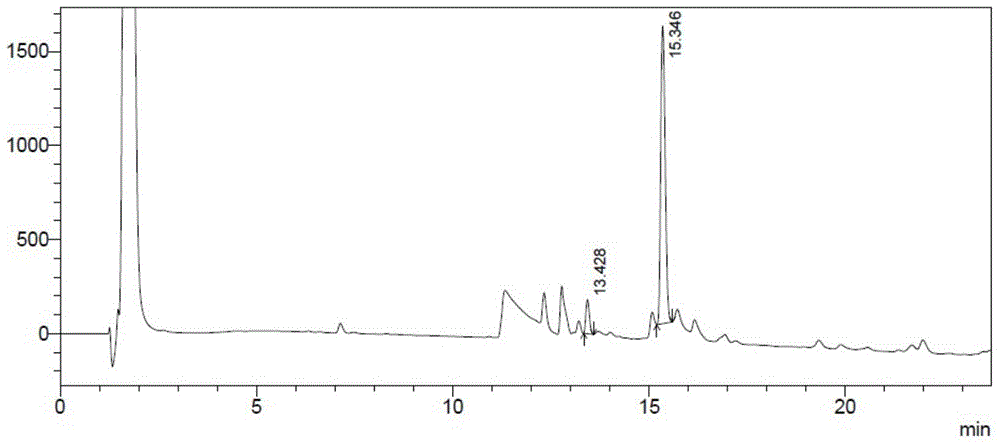
[0059] (2) Deletion of B30-insulin aspart coupling to obtain insulin aspart ester: Take the isoelectric point precipitate DesB30-insulin aspart and centrifuge, dissolve with 10ml 10% glacial acetic acid, the protein concentration is 100mg / ml, 15m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com