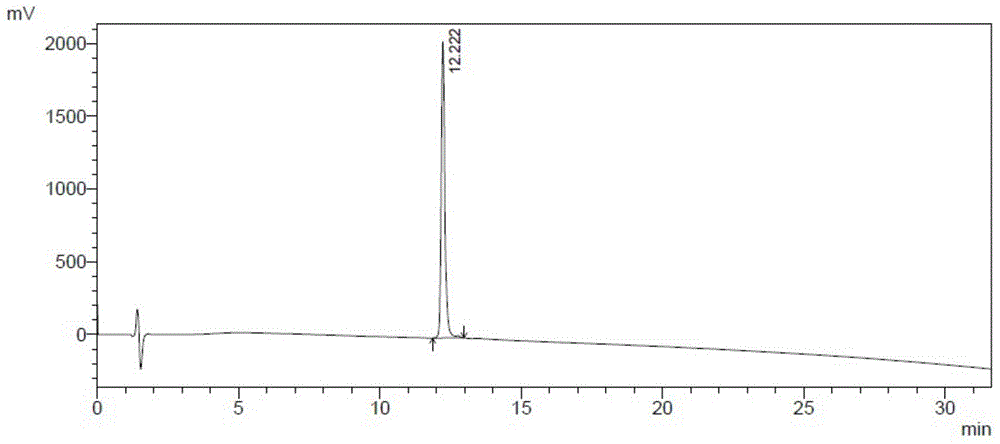
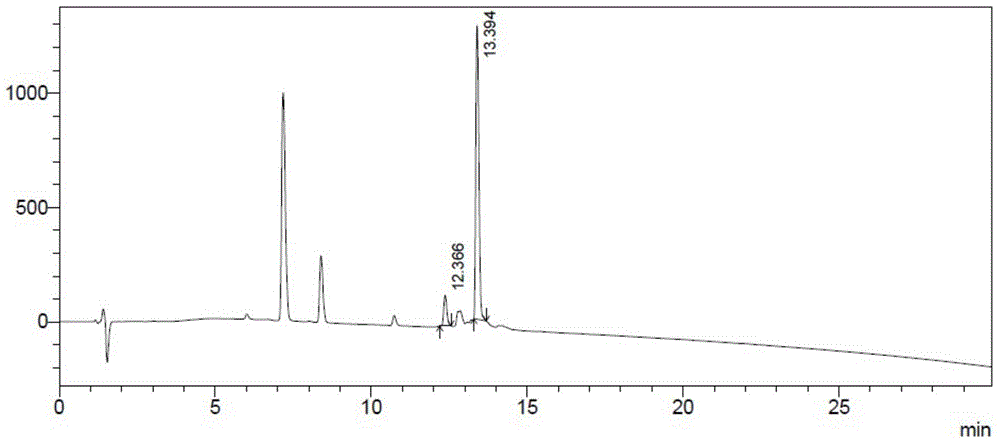
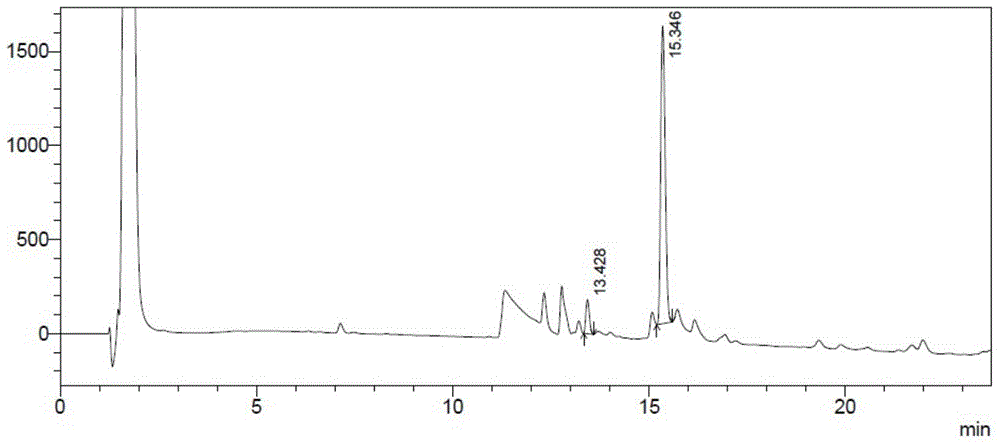
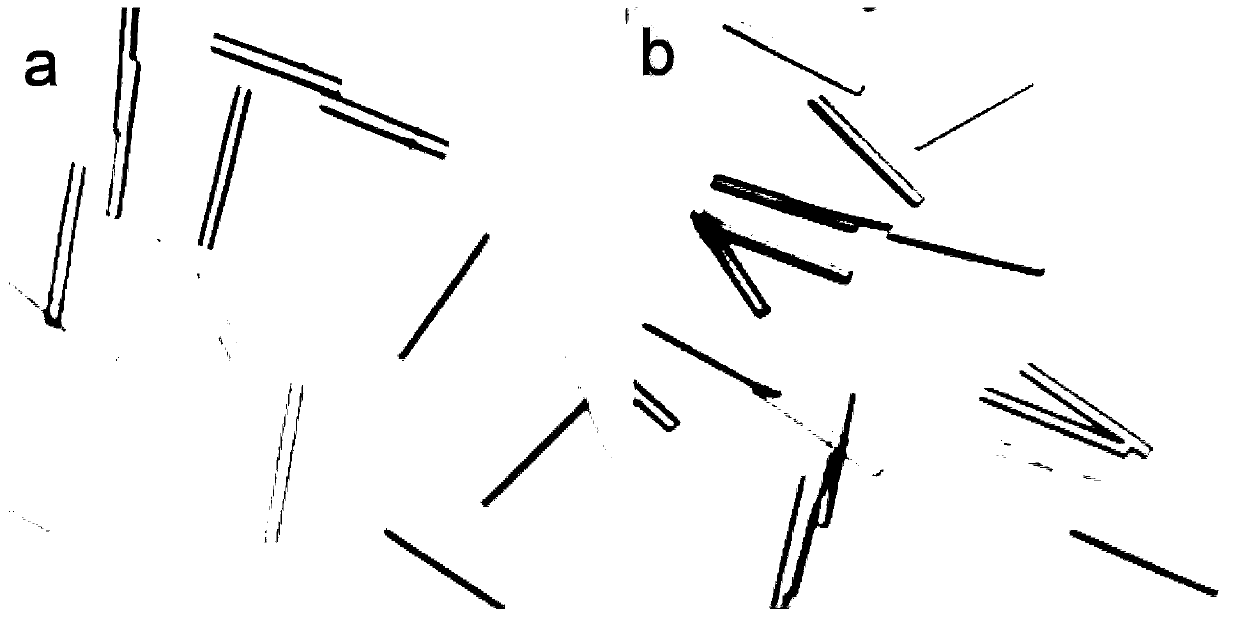
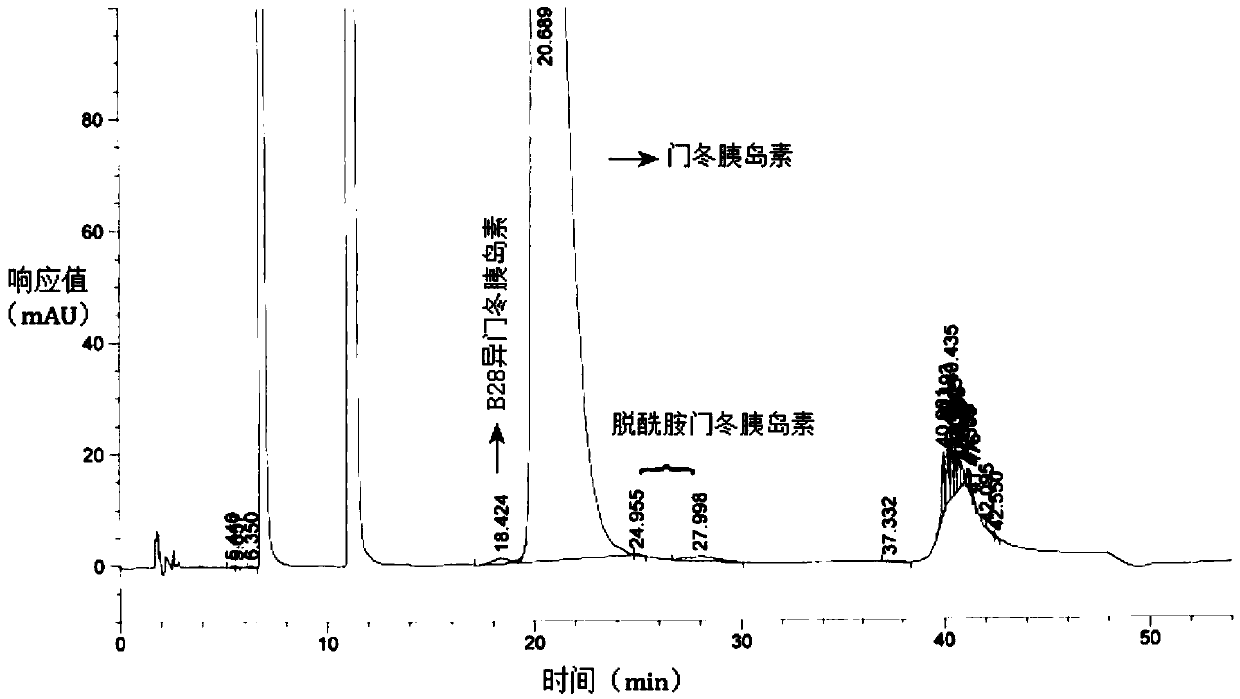
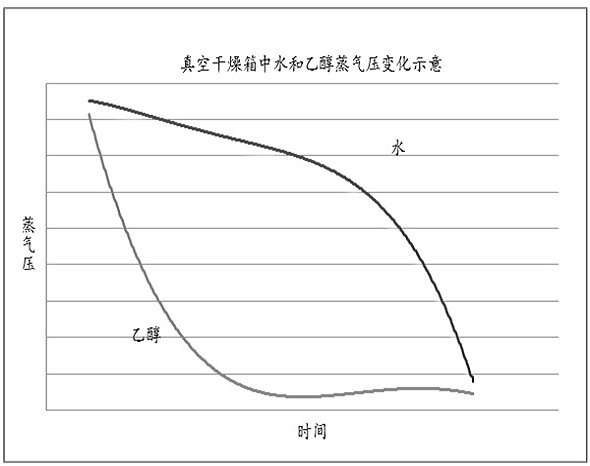
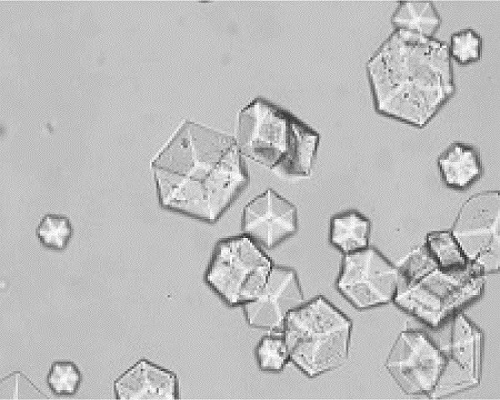
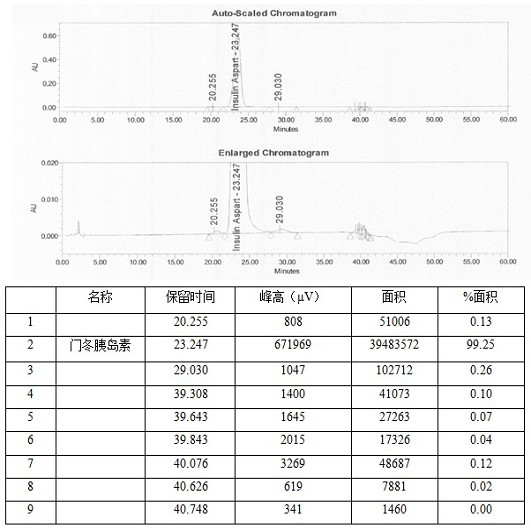
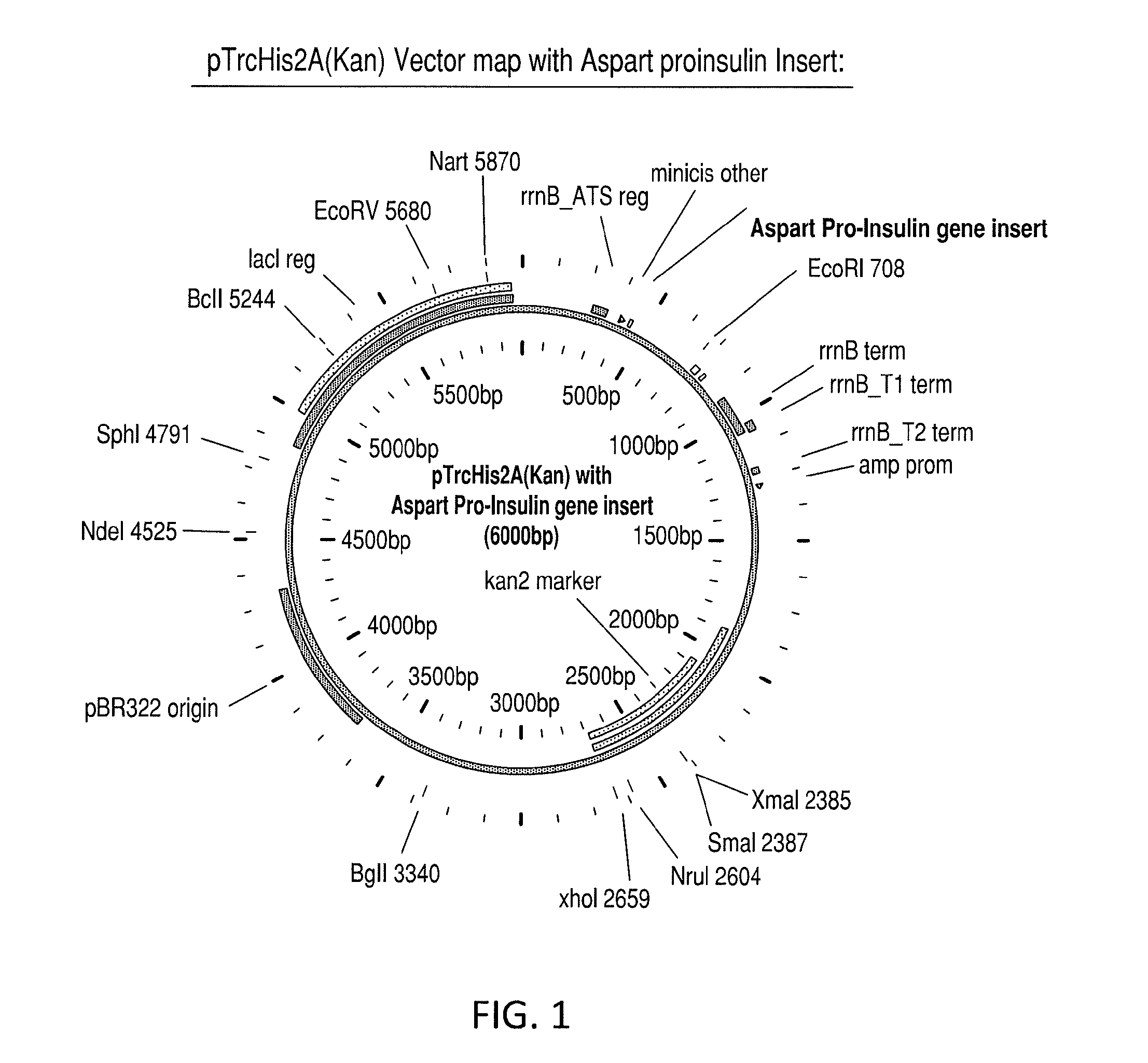
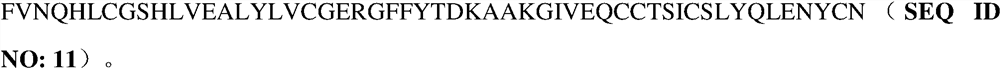Patents
Literature
31 results about "Insulin aspart" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Insulin aspart, sold under the brand name Novolog among others, is a type of manufactured insulin used to treat type I and type II diabetes. Typically it is taken just before eating. It is generally used by injection under the skin but may also be used by injection into a vein. Maximum effect occurs after about 2 hours and lasts for 4 hours. Generally a longer-acting insulin like NPH is also needed.
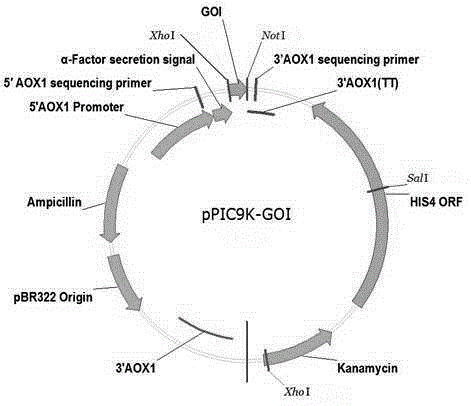
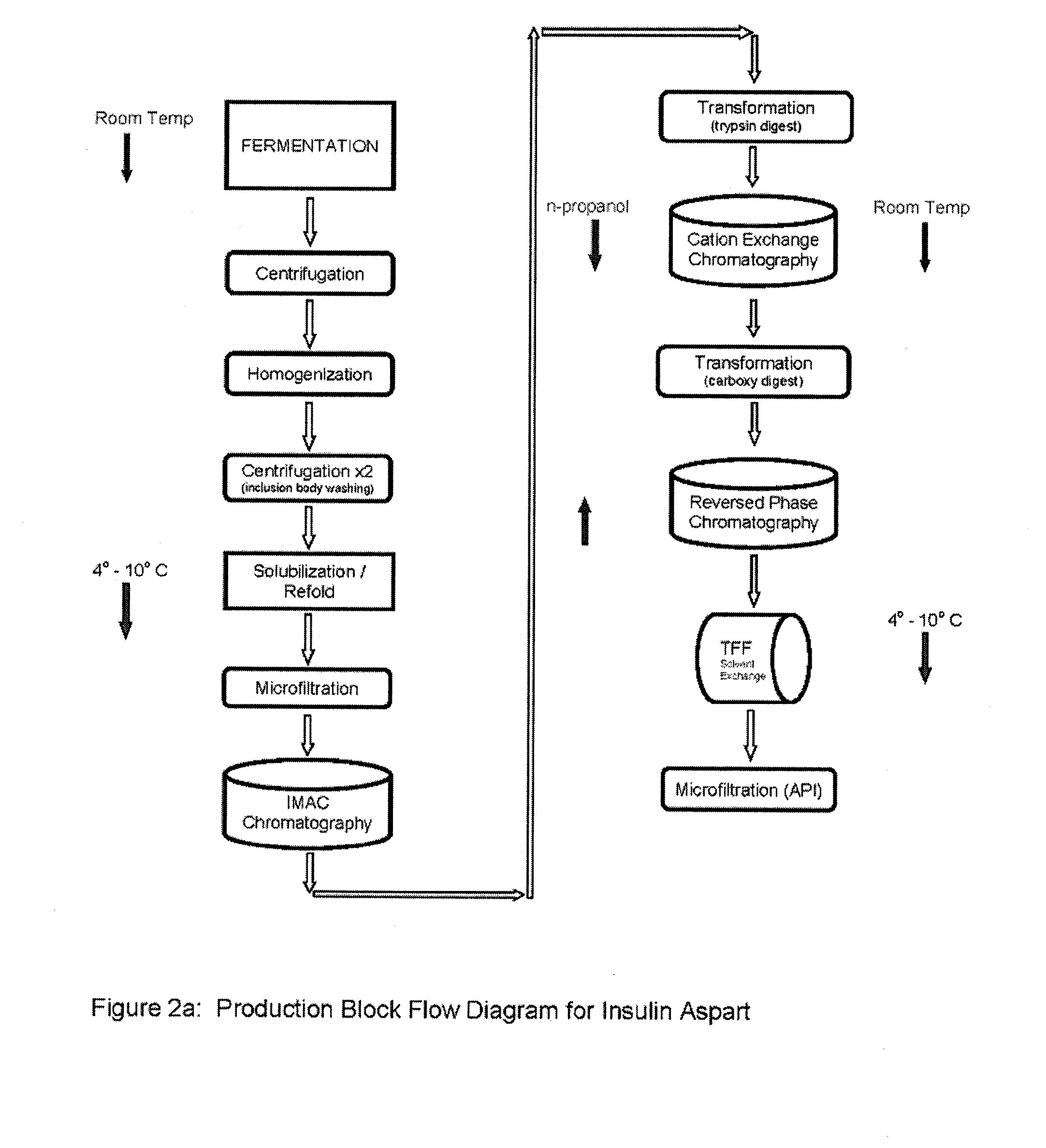
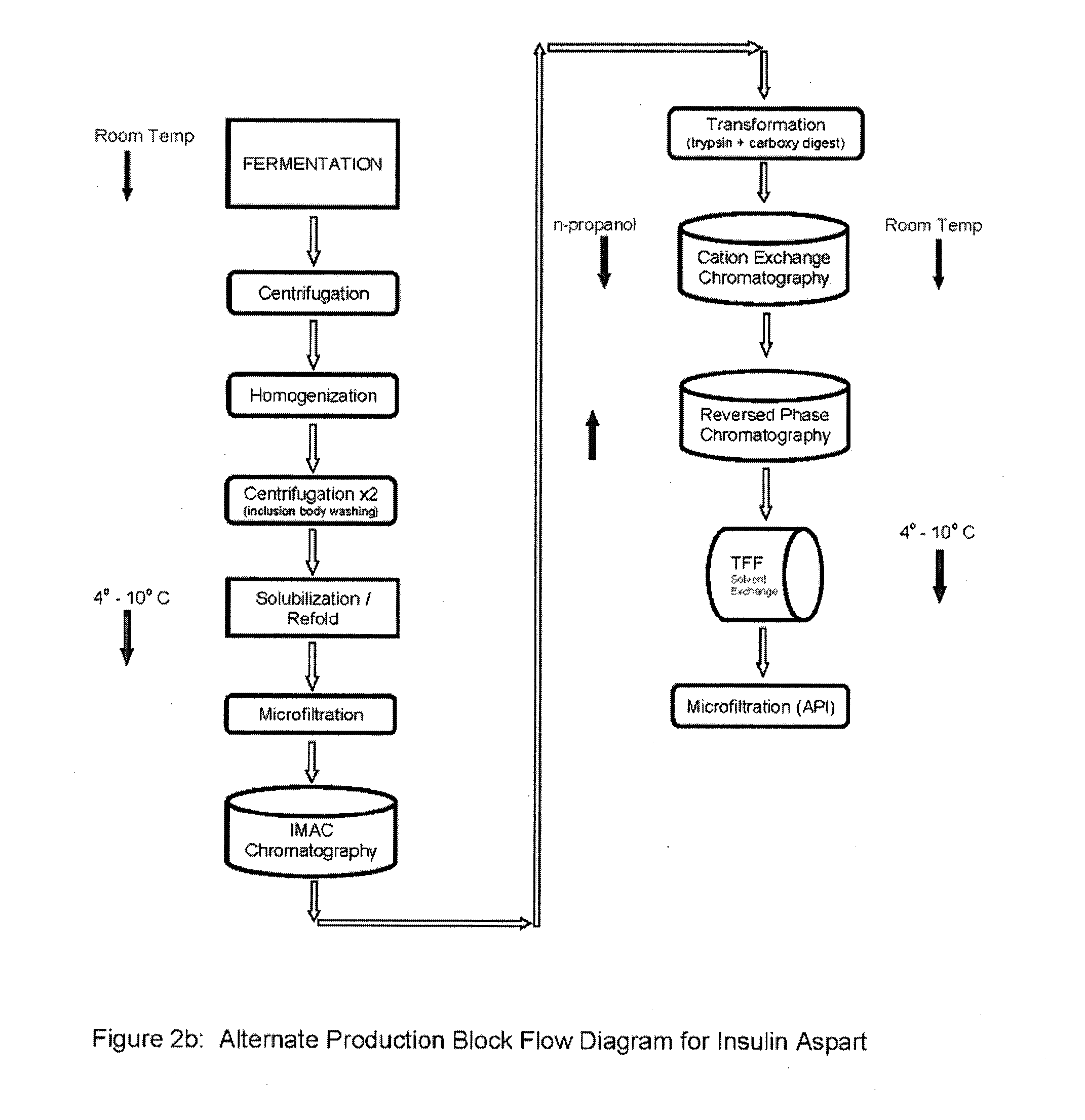
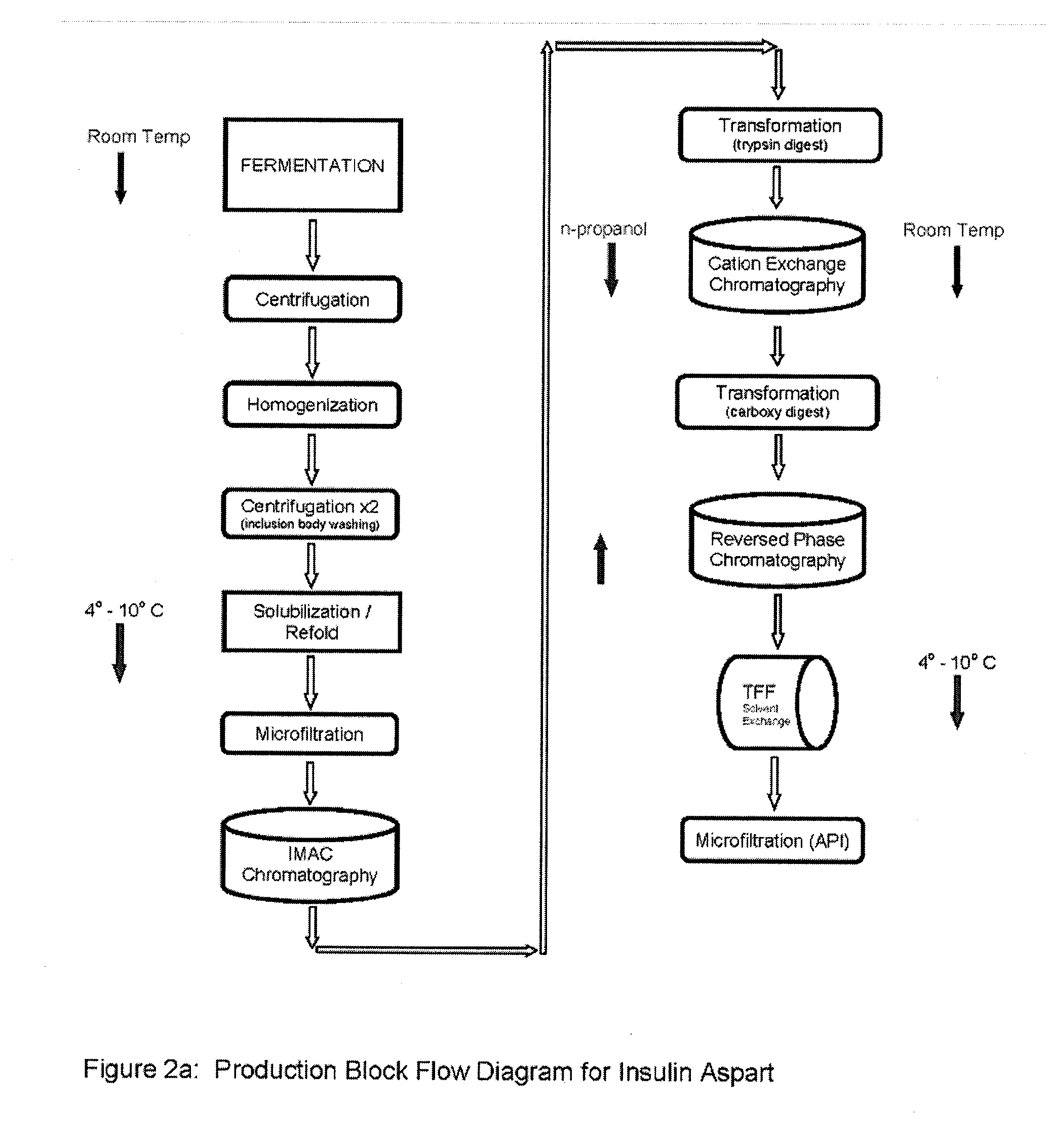
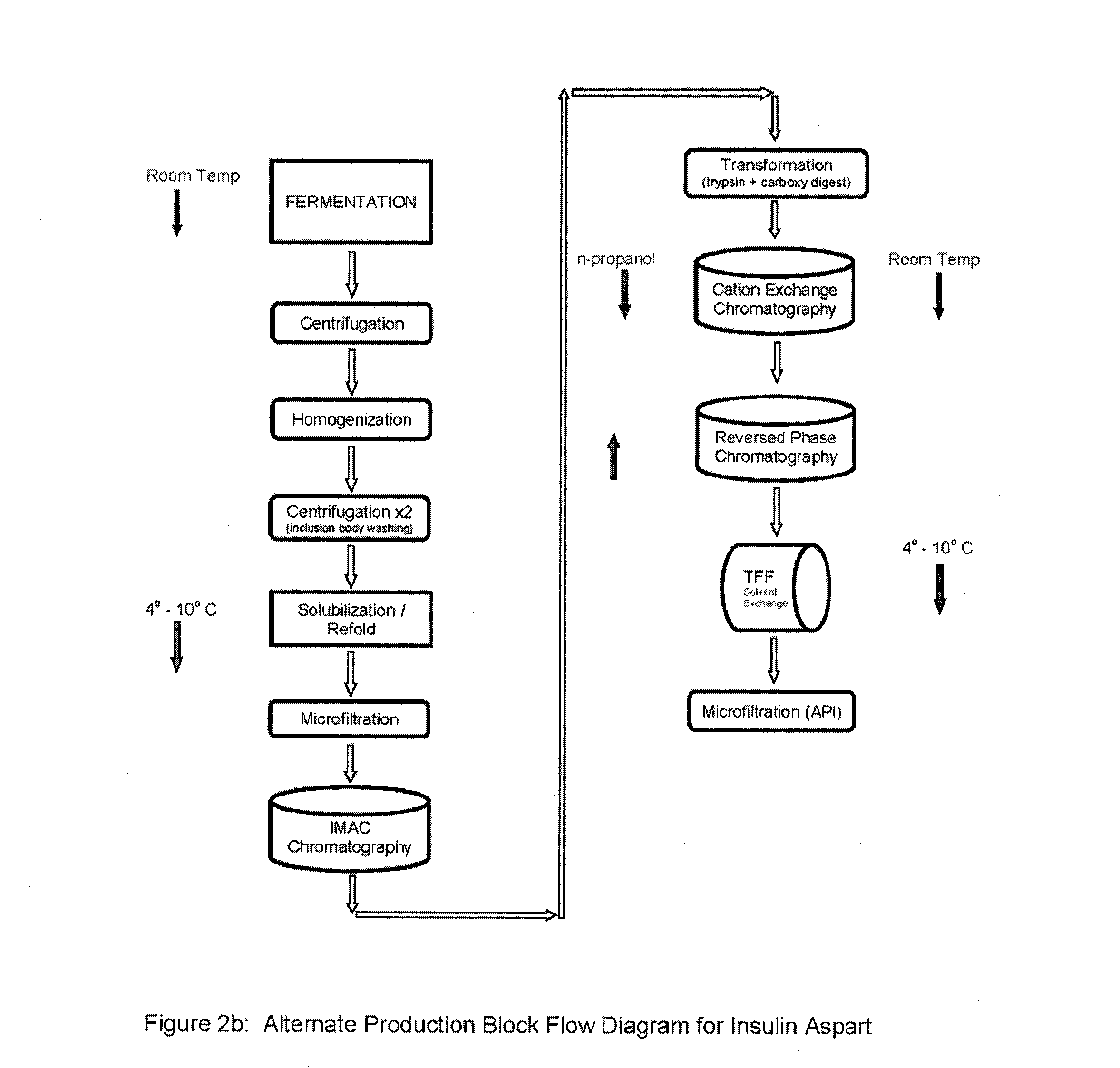
Preparation method for insulin aspart through recombinant expression by using yeast
ActiveCN105087724AImprove digestion efficiencyLow miscut rateMicroorganism based processesInsulinsEnzyme digestionThreonine
The invention discloses a preparation method for insulin aspart through recombinant expression by using yeast, and concretely relates to a preparation method for insulin aspart through recombinant expression by using pichia yeast. Concretely, the method comprises the following technological process: effectively secreting and expressing human aspart proinsulin, performing lysyl endopeptidase single enzyme digestion to obtain insulin aspart deleting B30, coupling with a threoninate, and performing deprotection, anti-phase purification and crystallization. The method is relatively suitable for industrialized preparation of recombinant insulin aspart.
Owner:CHONGQING PEG BIO BIOTECH CO LTD
Method for preparing stable insulin aspart crystal
The invention discloses a method for preparing stable insulin aspart crystal, and belongs to the field of purification and preparation of artificial insulin. The method comprises the following steps of: preparing an organic solvent, phenolic substance and crystalline liquid at 10-30 DEG C, wherein the organic solvent comprises 0.2-1.5M of glycine and 3.0-10.0g / L of recombined insulin aspart, the volume fraction content of the organic solvent is 10-30%, the volume fraction content of the phenolic substance is 0.2-0.4%, and the crystalline liquid comprises 0.2-0.5M of salt, regulating the pH value of the crystalline liquid to be 6.0-6.5, adding zinc ion, crystallizing for 3-6 hours, then cooling to 2-8 DEG C, standing for 12-18 hours, and obtaining the stable insulin aspart crystal. According to the method, the freeze drying time for products is shortened, the product stability is improved, and the method is applicable to commercial production of the recombined insulin aspart.
Owner:ZHUHAI UNITED LAB
Stabilized pharmaceutical formulations of insulin aspart
ActiveUS20150190475A1Peptide/protein ingredientsMetabolism disorderInsulin aspartPharmaceutical formulation
Owner:SANOFI SA
Quick-acting insulin aspart precursor protein and preparation method for quick-acting insulin
InactiveCN105418755AHigh expressionStable structureFungiMicroorganism based processesInsulin aspartInsulin Precursor
The invention provides an insulin aspart precursor. The amino acid sequence is Z1(XY)nZ2Z3Z4-B(1-29)-C1C2C3-A(1-21), wherein Z1, X and Z2 are Asp or Glu respectively, Y is Ala or Gly, n is an integer from 1-10, Z3 is Pro, Glu or Asp, Z4 and C3 are Lys or Arg respectively, C1 and C2 are Glu, Asp, Ala or Gly respectively, B(1-29) is insulin aspart B chain 1-29 bit amino acid, and A(1-21) is insulin aspart A chain 1-21 bit amino acid. The invention also provides a coding gene, a cloning vector or an expression vector containing the coding gene, a transformant, and a method for preparing quick-acting insulin by utilization of the quick-acting insulin aspart precursor protein. The expression level of the product is high, the fermentation expression product purity is high, the extraction method is simple and the yield is high.
Owner:ZHUHAI JINBAIKANG BIOLOGICAL TECH CO LTD
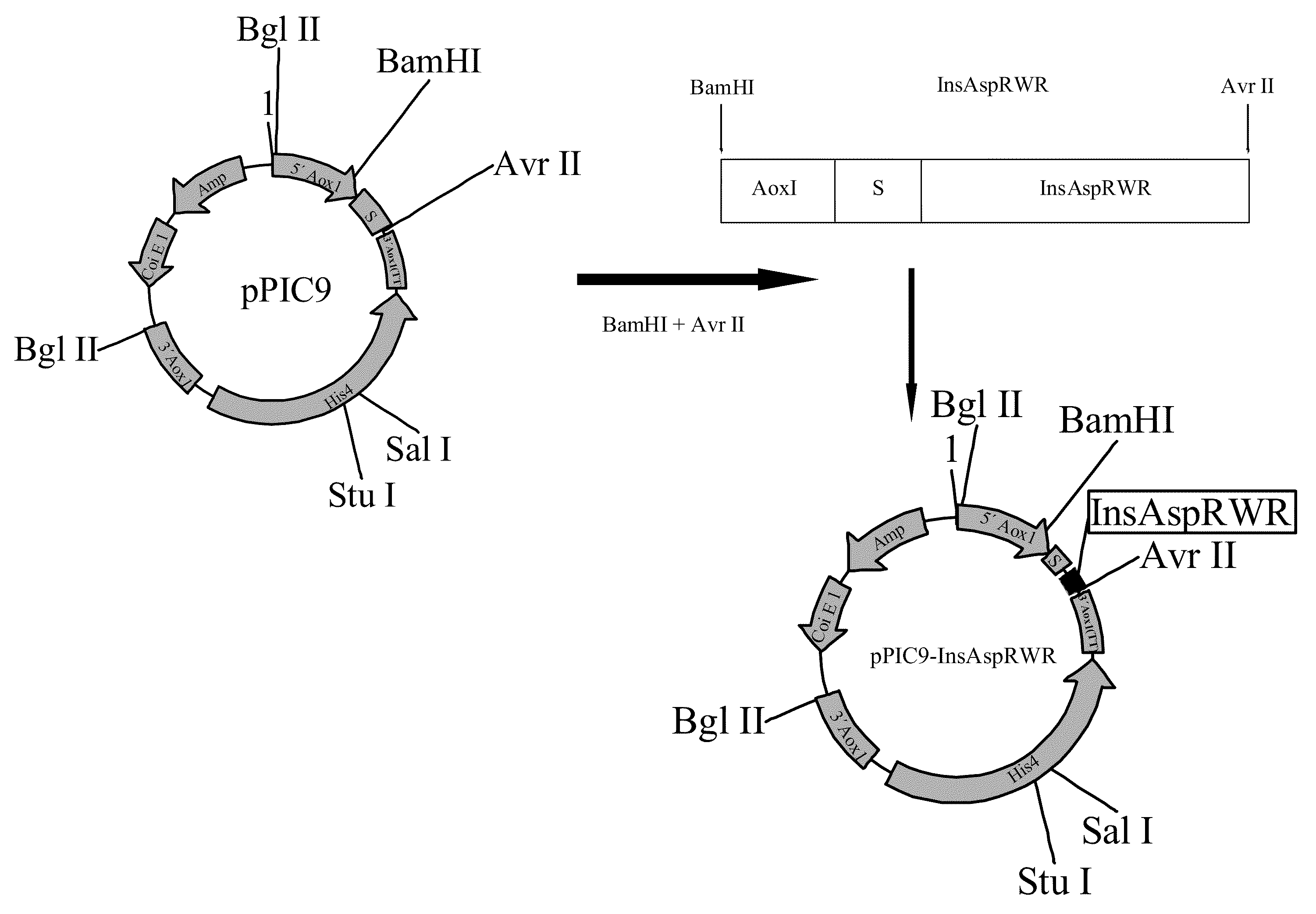
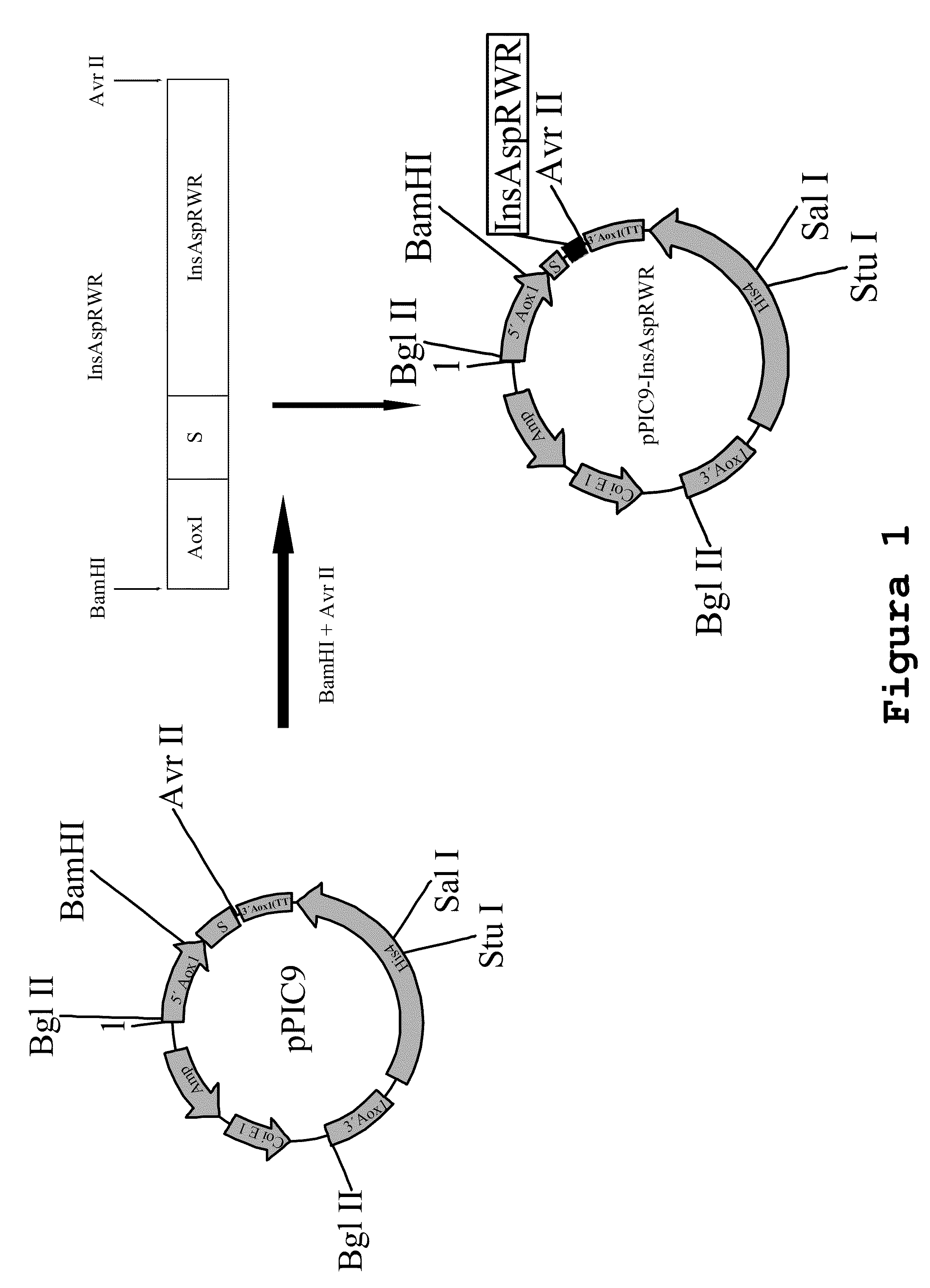
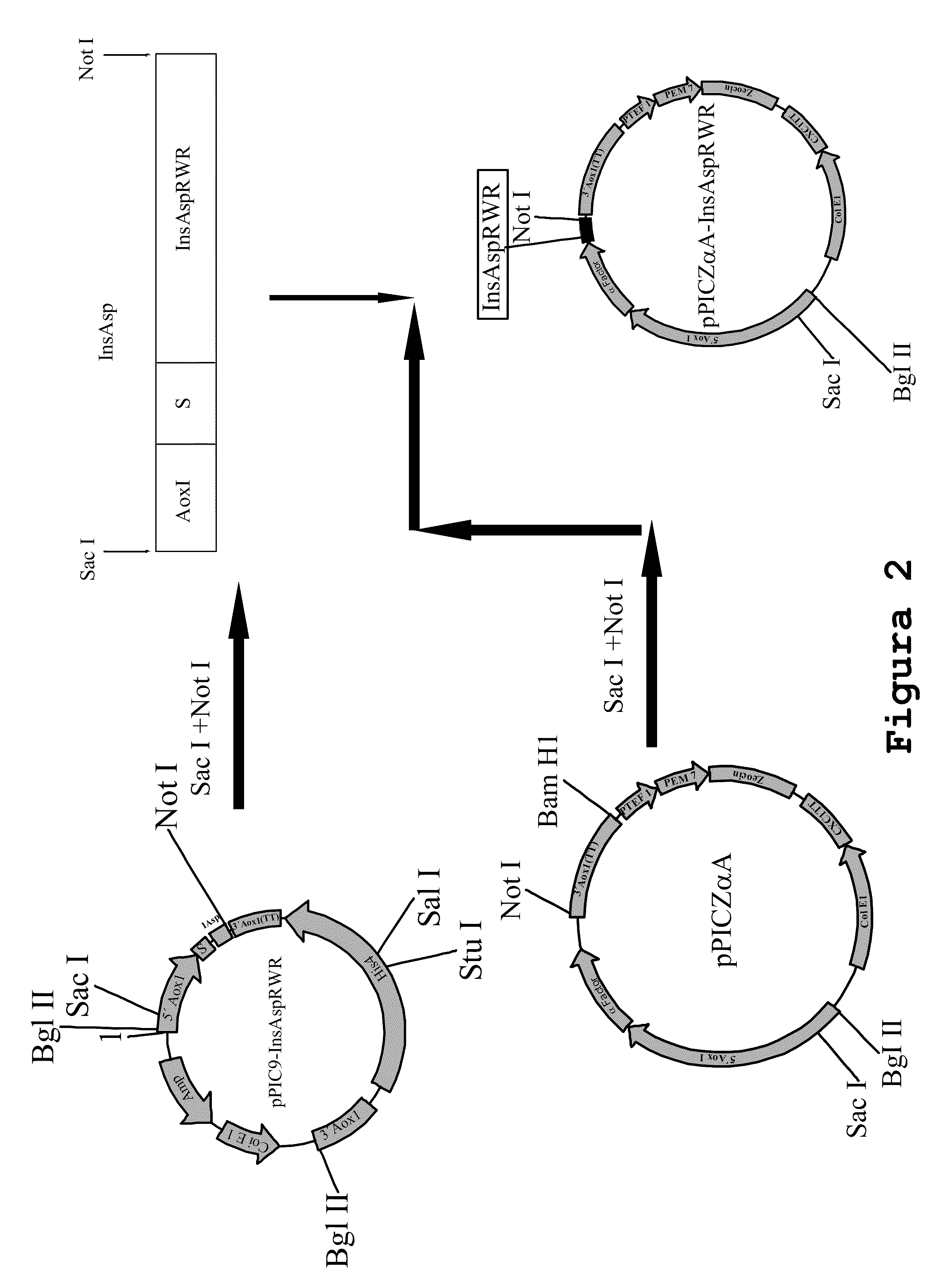
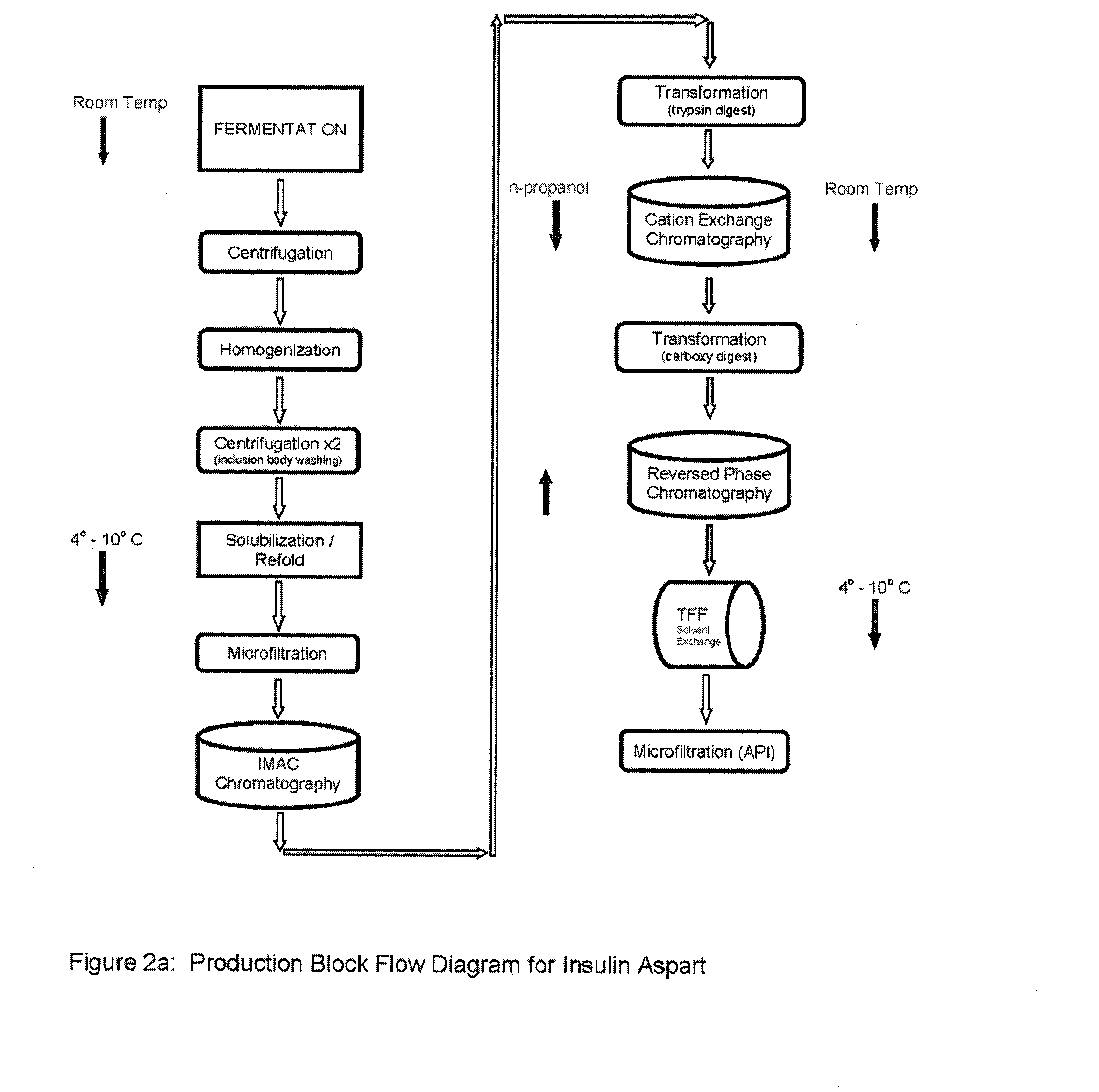
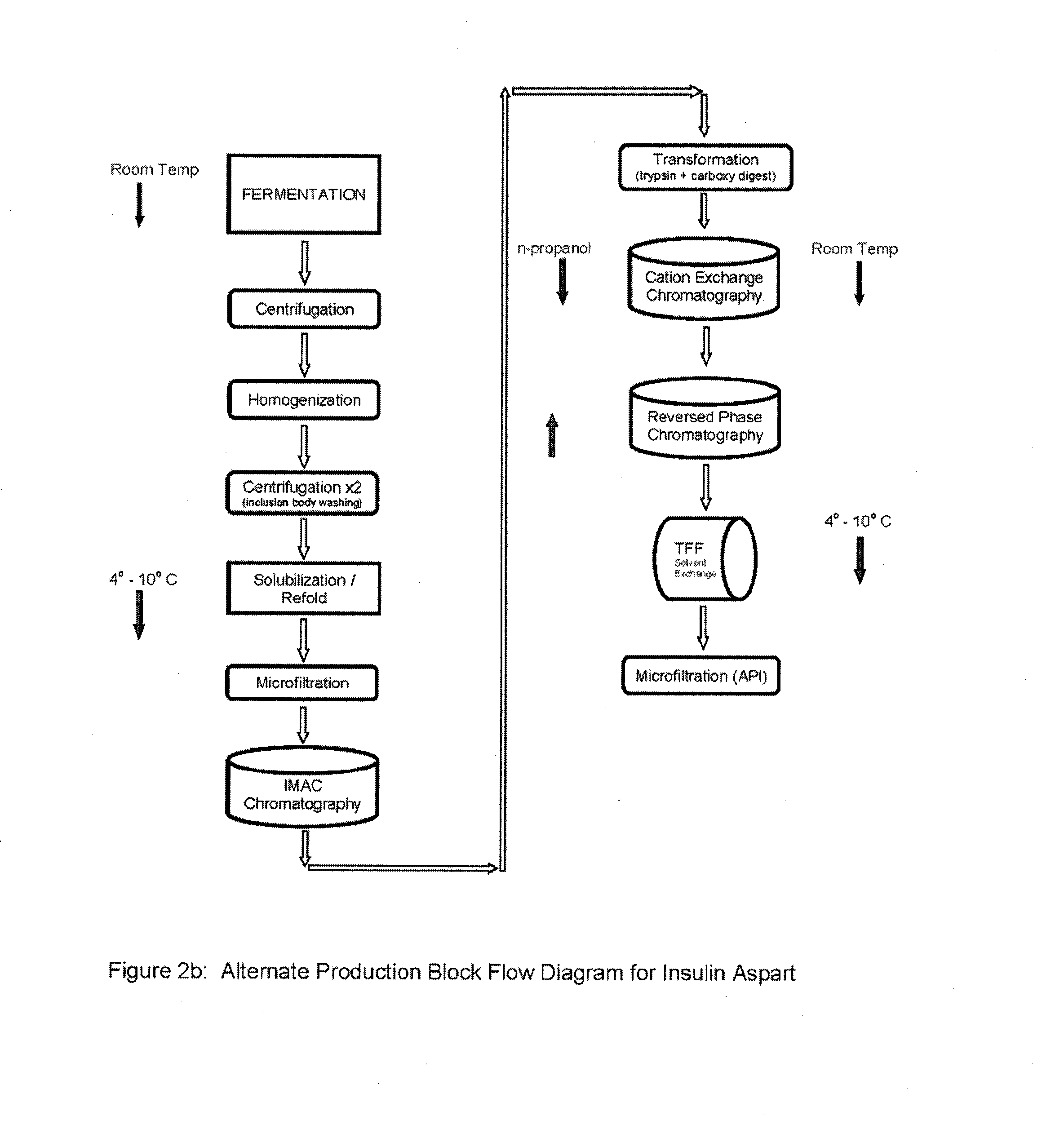
Process for obtaining aspart insulin using a pichia pastoris yeast strain
The present invention refers to a method for producing a human insulin analogue with high efficiency and excellent yield, by means of a biotechnological process comprising transformation of a Pichia pastoris yeast strain. In particular, the invention refers to a biotechnological process for obtaining aspart insulin.
Owner:LAB BETA SA
Stabilized pharmaceutical formulations of insulin aspart
ActiveUS9895423B2Peptide/protein ingredientsMetabolism disorderInsulin aspartPharmaceutical formulation
Owner:SANOFI SA
External biological activity determination method for human insulin and analog or conjugate
ActiveCN105092490AGood repeatabilityEasy to operateMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsPEGylated insulinDrug biological activity
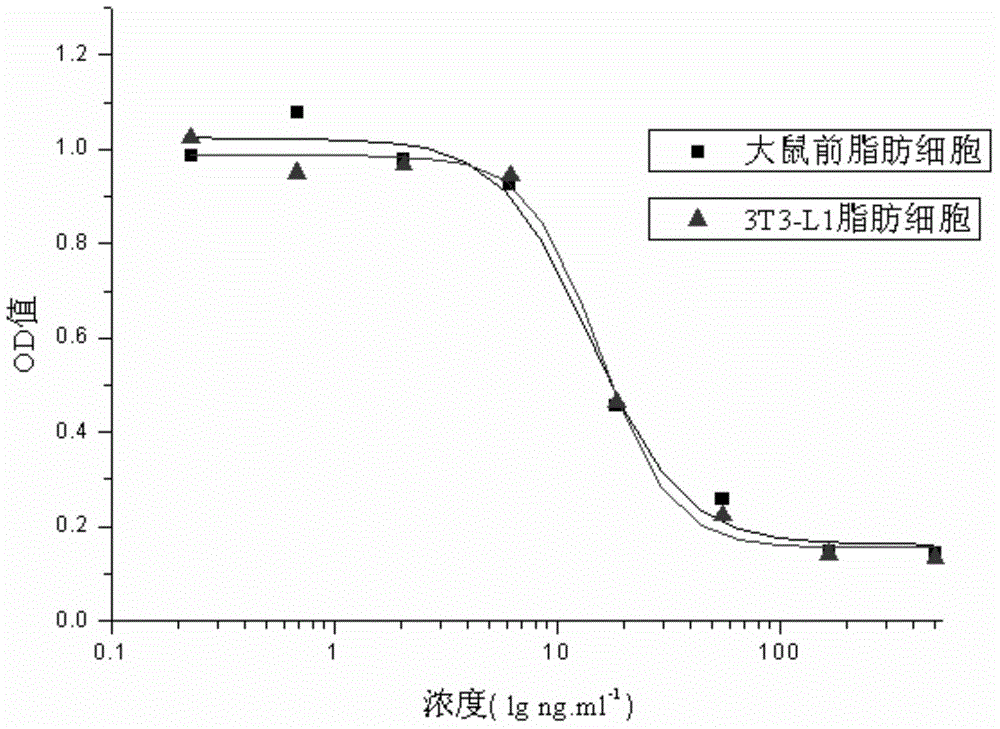
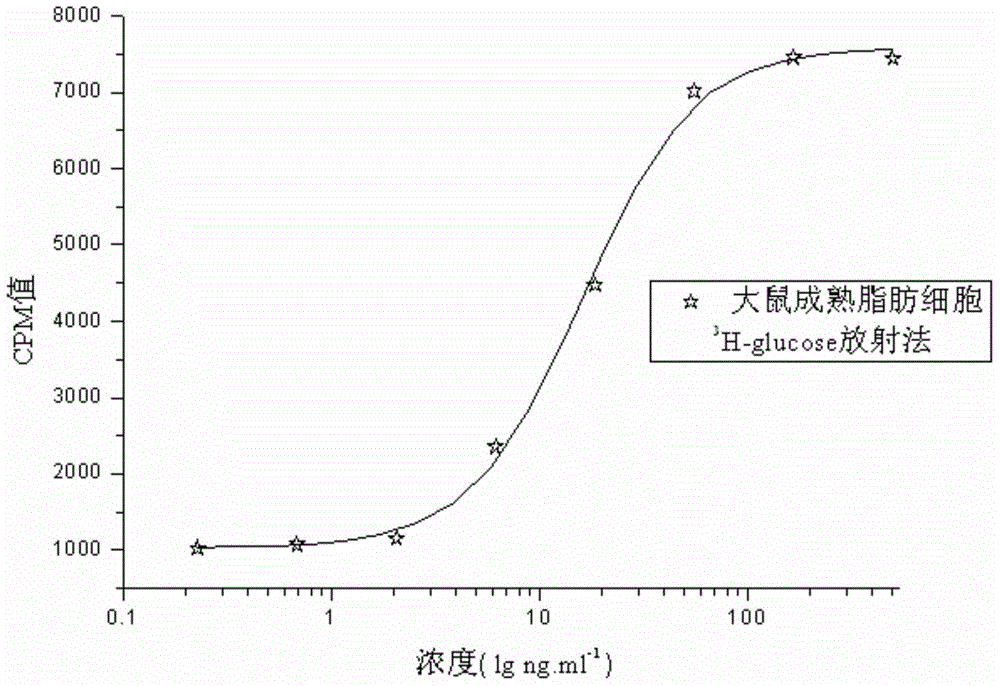
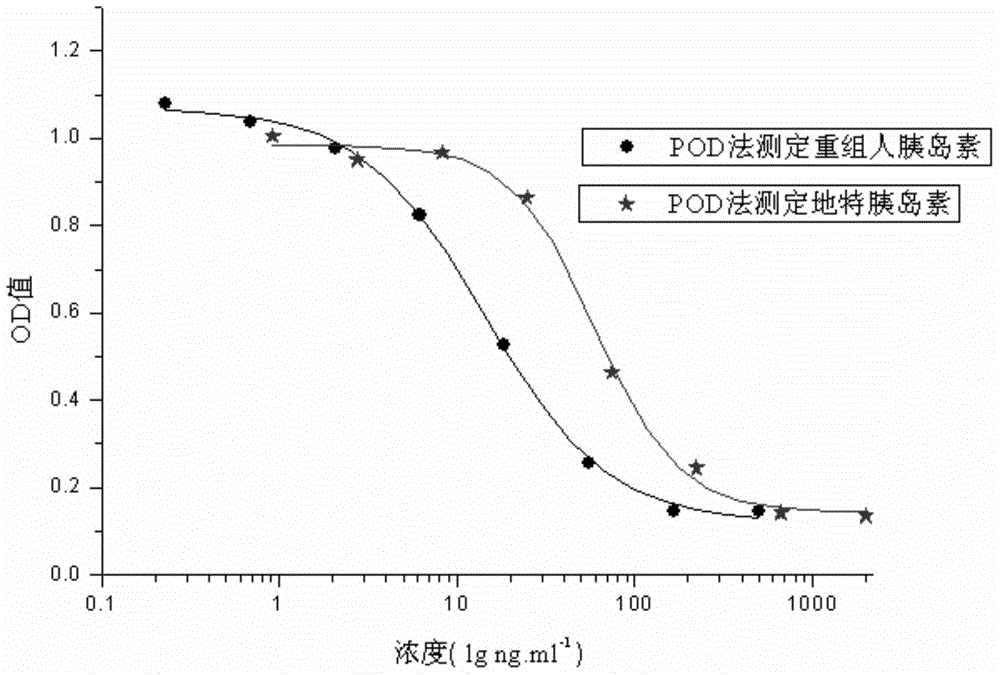
The invention relates to a human insulin and analog including rapid-acting insulin (e.g. Insulinaspart, InsulinLispro, and InsulinGlulisine), long-acting insulin (e.g. Insulinglargine, InsulinDetemir, and InsulinDegludec), and modified conjugate (e.g. PEG-Insulin and PEG conjugatedinsulin lispro ), and also relates to the biological value determination method for the cell culture in vitro of PEG-Insulin and application thereof. The external biological activity determination method for human insulin and analog or conjugate is induced in vitro into adipocyte by using preadipocyte of male rat, and measure the glucose consumption after insulin reacting in the adipocyte nutrient solution by using glucose oxidase method to calculate the corresponding insulin biological value.
Owner:CHONGQING PEG BIO BIOTECH CO LTD
Method for preparing stable insulin aspart crystal
The invention discloses a method for preparing stable insulin aspart crystal, and belongs to the field of purification and preparation of artificial insulin. The method comprises the following steps of: preparing an organic solvent, phenolic substance and crystalline liquid at 10-30 DEG C, wherein the organic solvent comprises 0.2-1.5M of glycine and 3.0-10.0g / L of recombined insulin aspart, the volume fraction content of the organic solvent is 10-30%, the volume fraction content of the phenolic substance is 0.2-0.4%, and the crystalline liquid comprises 0.2-0.5M of salt, regulating the pH value of the crystalline liquid to be 6.0-6.5, adding zinc ion, crystallizing for 3-6 hours, then cooling to 2-8 DEG C, standing for 12-18 hours, and obtaining the stable insulin aspart crystal. According to the method, the freeze drying time for products is shortened, the product stability is improved, and the method is applicable to commercial production of the recombined insulin aspart.
Owner:ZHUHAI UNITED LAB
Fast-acting insulin preparation and preparation method thereof
InactiveCN108096185AImprove antioxidant capacityReduced deamidationPeptide/protein ingredientsMetabolism disorderAntioxidantColor changes
The invention discloses application of L-histidine and pharmaceutically-acceptable derivatives thereof in improvement of stability of fast-acting insulin preparation. The fast-acting insulin preparation is Insulin Aspart. Shown by experiments, the L-histidine and the pharmaceutically-acceptable derivatives thereof serve as a stabilizer and an antioxidant in injections, deamidization, peptide chaincracking and polymerization reactions of the Insulin Aspart are reduced, the oxidation resistance of Insulin Aspart injections is improved, and the Insulin Aspart injections are prevented from oxidated destruction even liquid medicine color change during storage. The preparation process is simple and convenient and is applicable to industrial and big production.
Owner:ZHUHAI JINBAIKANG BIOLOGICAL TECH CO LTD
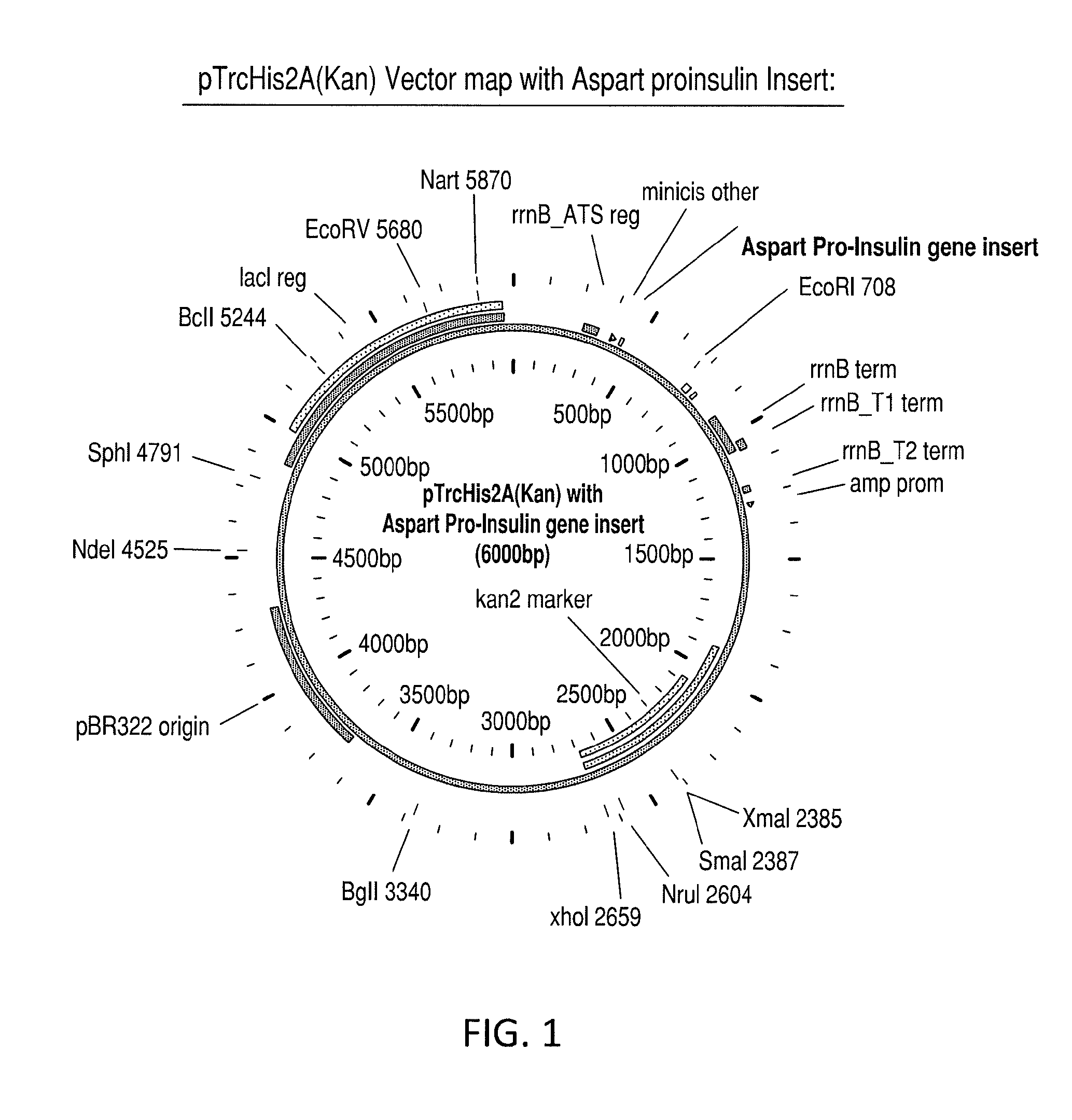
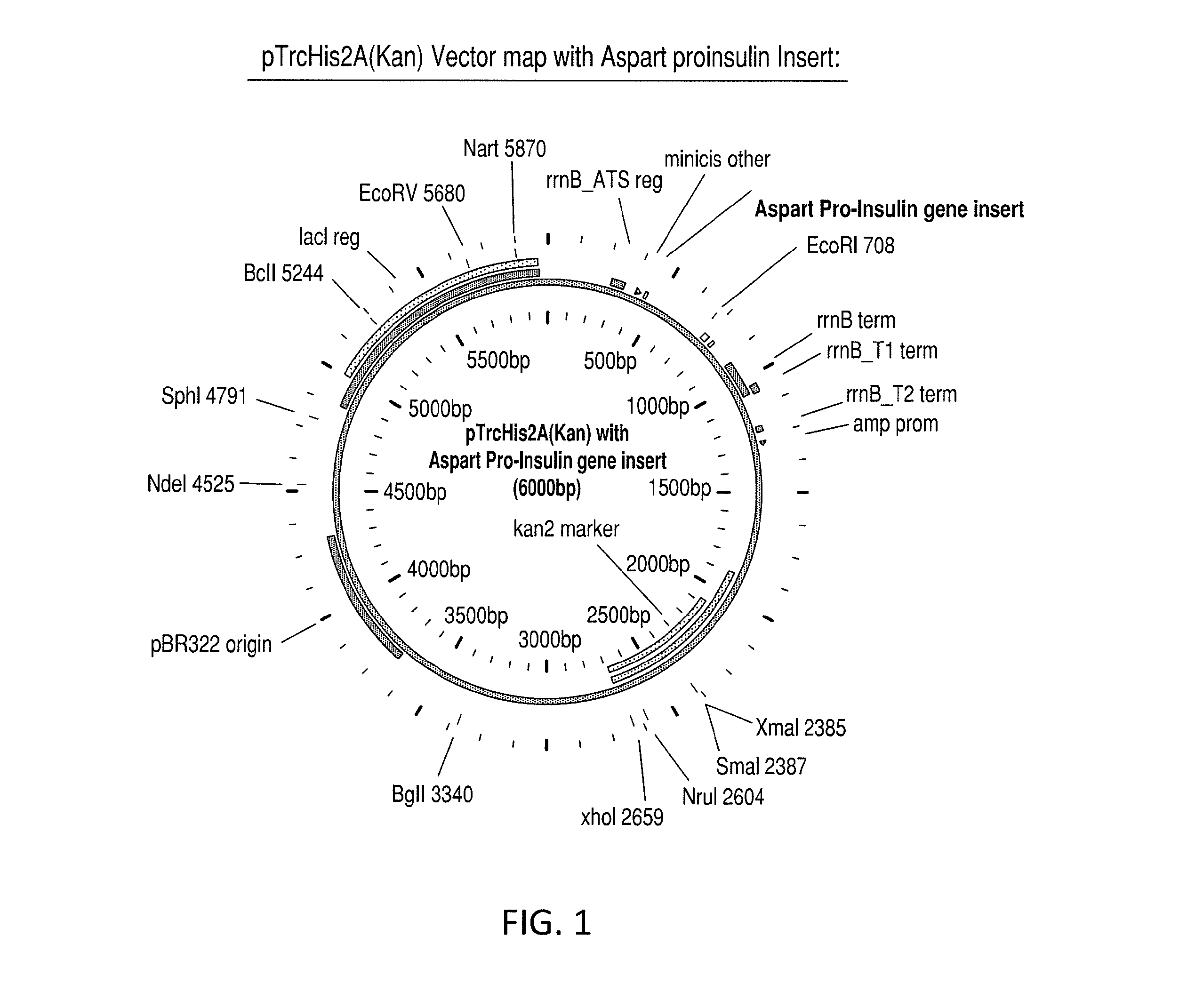
Aspart proinsulin compositions and methods of producing aspart insulin analogs therefrom
Aspart modified proinsulin sequences that have a modified C-peptide amino acid and / or nucleic acid modification for producing aspart insulin analogs are provided. Highly efficient processes for preparing the aspart insulin analogs and improved preparations containing the aspart insulin analogs prepared according to the methods described herein are also provided.
Owner:ELONA BIOTECH
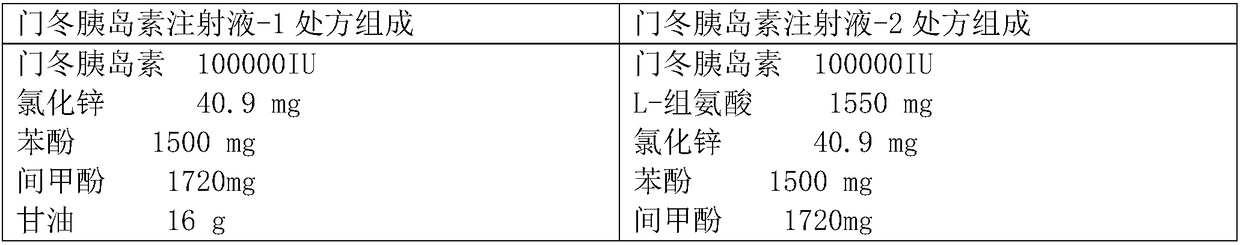
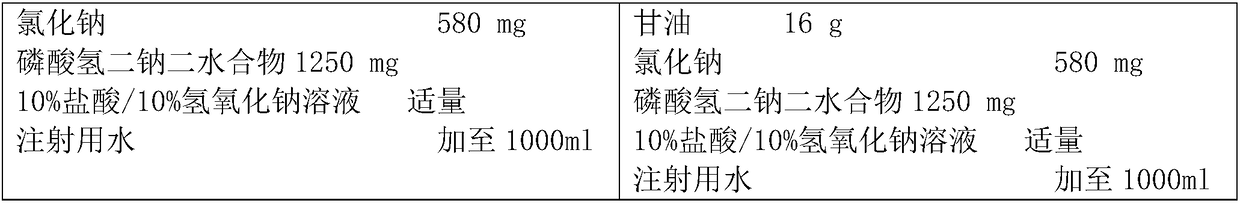
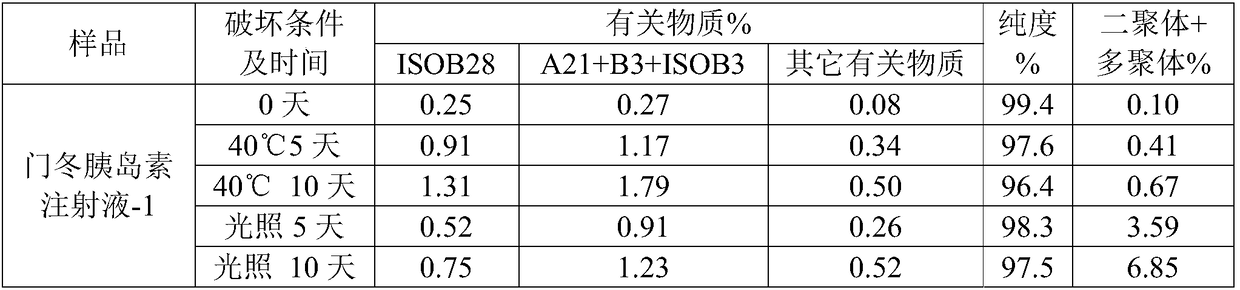
Preparation method of insulin aspart injection
ActiveCN108114270AThe process steps are simpleImprove stabilityPeptide/protein ingredientsMetabolism disorderInsulin injectionMedicine
The invention provides a preparation method of an insulin aspart injection, relating to the field of pharmaceutical preparations, and aims to provide the preparation method of the insulin aspart injection simple to operate, safe and reliable and stable in quality. The method comprises the following steps: a) providing an alkaline buffer solution; b) providing a mixed solution of a phenol mixture,an isoosmotic adjusting agent and a stabilizer; c) uniformly mixing the solution; d) adding insulin aspart and uniformly mixing the insulin aspart with the solution; or the method comprises the following steps: a') providing the alkaline buffer solution; b') adding insulin aspart to be mixed and dissolved; c') providing the mixed solution of the phenol mixture, the isoosmotic adjusting agent and the stabilizer; and d') uniformly mixing the solution.
Owner:NANJING HANXIN PHARMA TECH CO LTD
Compositions and method for enhancing insulin activity
A new treatment for individuals suffering from type 2 diabetes. The formulation used in the treatment includes a mixture of a thiazoleineione, an insulin, cinnamon bark extract, and at least one synergistic supplement selected from the group consisting of blueberry leaf extract, cranberry extract, kelp extract, sugar sea beet extract, acerola berry extract; ginger root extract, black cherry extract, green tea extract, Irish moss extract, aloe vera extract, and Stevia leaf extract. The thiazoleineione is preferably pioglitazone. The insulin is preferably an Insulin Aspart of rDNA origin. It is preferred that the cinnamon bark extract and synergistic supplement be in the form of a liquid concentrate.
Owner:STOJAN CURTIS C
Method for efficiently removing residual organic solvent in insulin aspart
ActiveCN113074519AImprove product qualityDrying solid materials without heatDrying machines with local agitationOrganosolvInsulin aspart
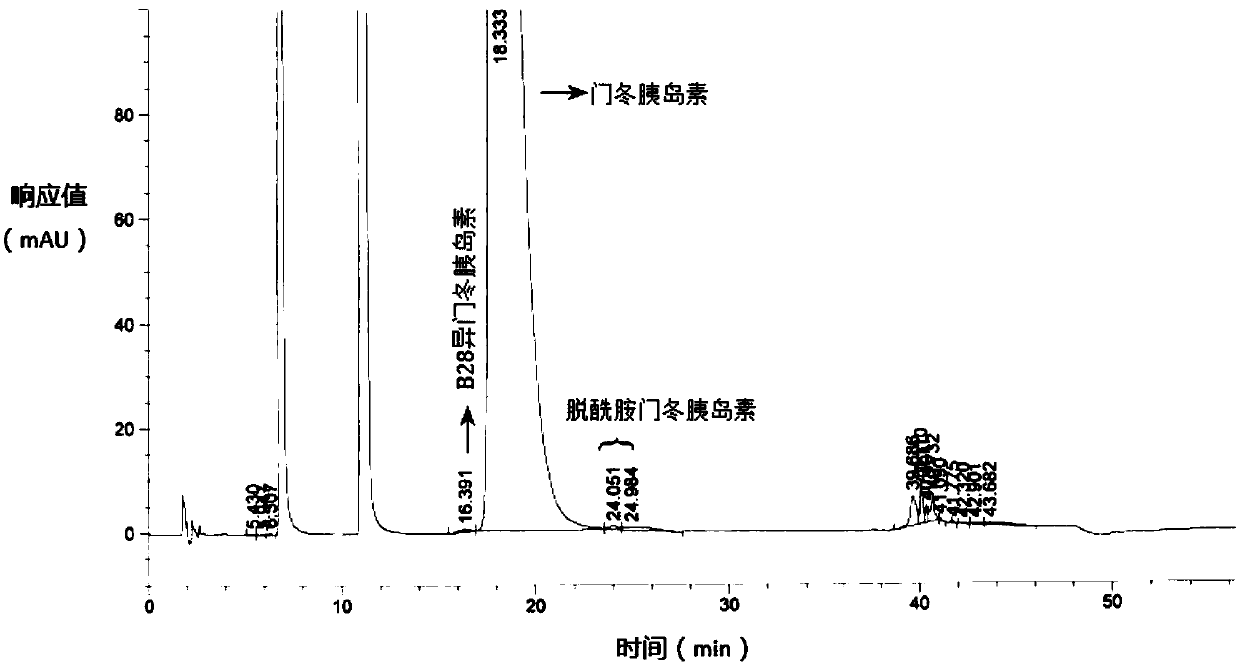
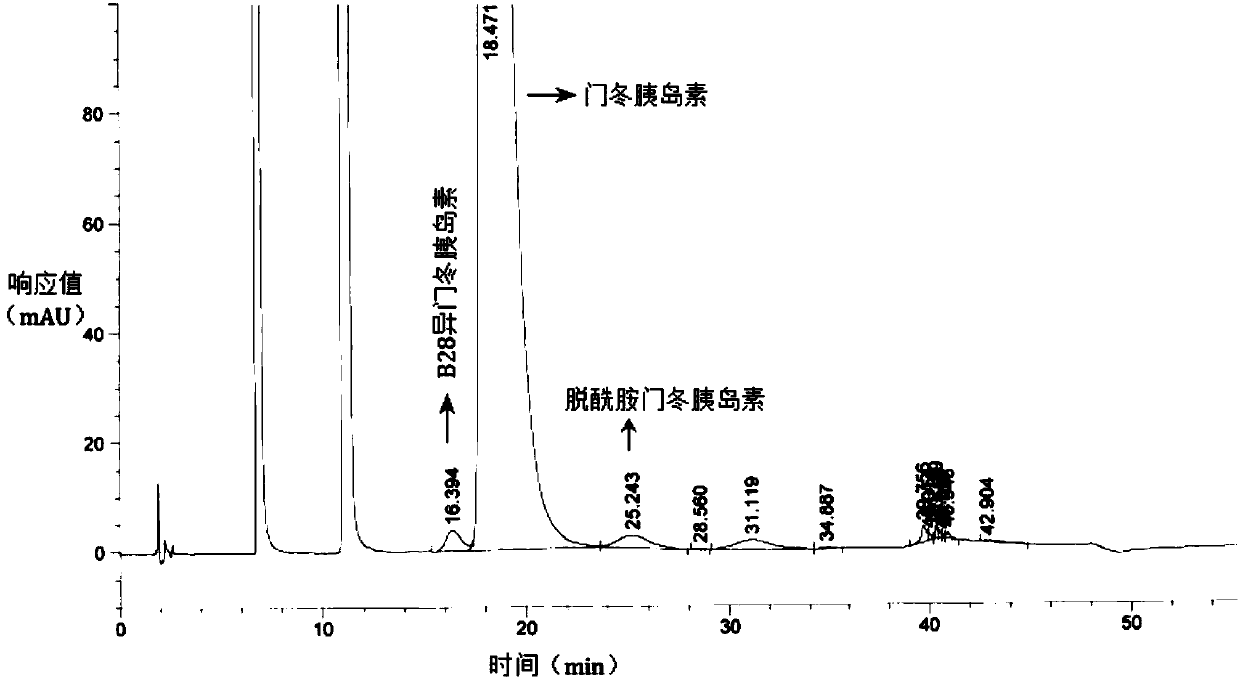
The invention relates to the field of biological medicine, in particular to a method for efficiently removing solvent residues in the drying process of insulin aspart crystals. According to the insulin aspart crystal drying process, primary vacuumizing is adopted to remove most of organic solvents; then water is added to preserve moisture and pressure, and the temperature and material turning over are controlled at proper time and during the period, the solvent crystal of the insulin aspart is converted into crystal water; then vacuumizing is conducted, the temperature is controlled and material turning over is conducted at proper time, so that the residual organic solvent which is difficult to remove is further removed; and the steps of moisturizing, pressure maintaining and vacuumizing are repeated, so that the wet solid is in full contact with the water vapor, and the stability of a sample is ensured on the basis of reducing the solvent residue. According to the method, the ethanol residual solvent in a kilogram-level insulin finished product prepared by the method reaches 3000 ppm or below, the purity of the product is reaches 99% or above, and the problems of high solvent residue, unstable quality, high equipment requirement, high amplification cost and unsuitability for large-scale production in the prior art are solved.
Owner:AMPHASTAR NANJING PHARMA
Aspart Proinsulin Compositions and Methods of Producing Aspart Insulin Analogs
Aspart modified proinsulin sequences that have a modified C-peptide amino acid and / or nucleic acid modification for producing aspart insulin analogs are provided. Highly efficient processes for preparing the aspart insulin analogs and improved preparations containing the aspart insulin analogs prepared according to the methods described herein are also provided.
Owner:AGILA BIOTECH PVT LTD
Insulin aspart crystallization process
ActiveCN113248591AOvercoming inefficiencyOvercoming the shortcomings of quantitative crystalline insulin aspartPeptide preparation methodsInsulinsOrganosolvGlycerol
The invention provides a preparation method and application of an insulin aspart crystal. According to the crystallization method disclosed by the invention, no organic solvent is used, the crystal is prepared by mixing two solutions, and the stable insulin aspart crystal is obtained from a crystallization solution containing insulin aspart, glycerol, phenol, m-cresol, sodium chloride, disodium hydrogen phosphate dihydrate, zinc chloride and protamine sulfate by optimizing a crystallization system. The insulin aspart crystal prepared by the method is regular in shape and uniform in size, and the preparation method is high in production efficiency and convenient for industrial application.
Owner:BEIJING HUIZHIHENG BIOTECHNOLOGY CO LTD +1
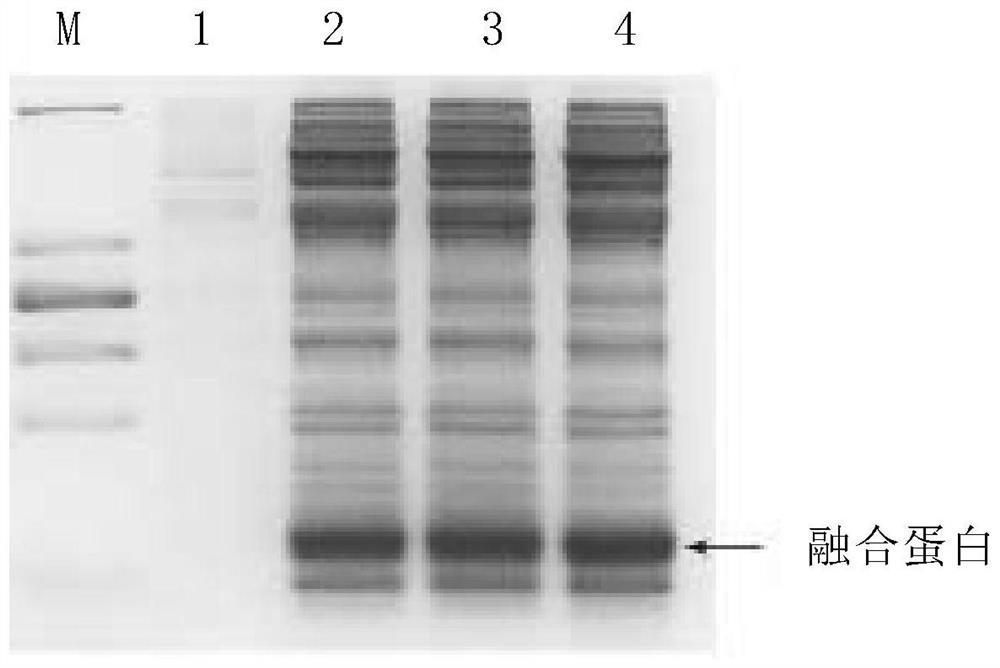
Fermentation culture method of recombinant escherichia coli for producing insulin aspart fusion protein
InactiveCN113980879AHigh expressionIncrease productivityPolypeptide with localisation/targeting motifBacteriaEscherichia coliYeast Proteins
The invention provides a culture medium suitable for fermenting recombinant escherichia coli to produce insulin aspart fusion protein. Specifically, the invention provides the culture medium containing yeast peptone, yeast extract powder, glycerol, Boc-L-lysine, a buffering agent and trace elements. The invention further provides a method for producing the insulin aspart fusion protein by fermentation of the culture medium. When the culture medium is used for fermentation, high bacterial density and high insulin aspart fusion protein yield can be obtained, the fermentation culture time is short, raw materials are easy to obtain, the virus carrying risk is avoided, the cost is low, and the culture medium is suitable for industrial production.
Owner:NINGBO KUNPENG BIOTECH CO LTD
A kind of insulin aspart crystallization process
ActiveCN113248591BIncrease productivitySuitable for industrial production needsPeptide preparation methodsInsulinsInsulin aspartOrganosolv
The invention provides a preparation method and application of insulin aspart crystal. The crystallization method of the present invention does not use organic solvents, and prepares crystals by mixing two solutions, and by optimizing the crystallization system, it contains insulin aspart, glycerin, phenol, m-cresol, sodium chloride, disodium hydrogen phosphate dihydrate, In the crystallization solution of zinc chloride and protamine sulfate, stable insulin aspart crystals were obtained. The insulin aspart crystals prepared by the method of the invention have regular shapes and uniform sizes, and the preparation method has high production efficiency and is convenient for industrial application.
Owner:BEIJING HUIZHIHENG BIOTECHNOLOGY CO LTD +1
Structure of a novel proinsulin aspart and method for preparing insulin aspart
ActiveCN112584853BReduce quality lossPromote maturityPeptide/protein ingredientsOxidoreductasesInclusion bodiesPancreatic hormone
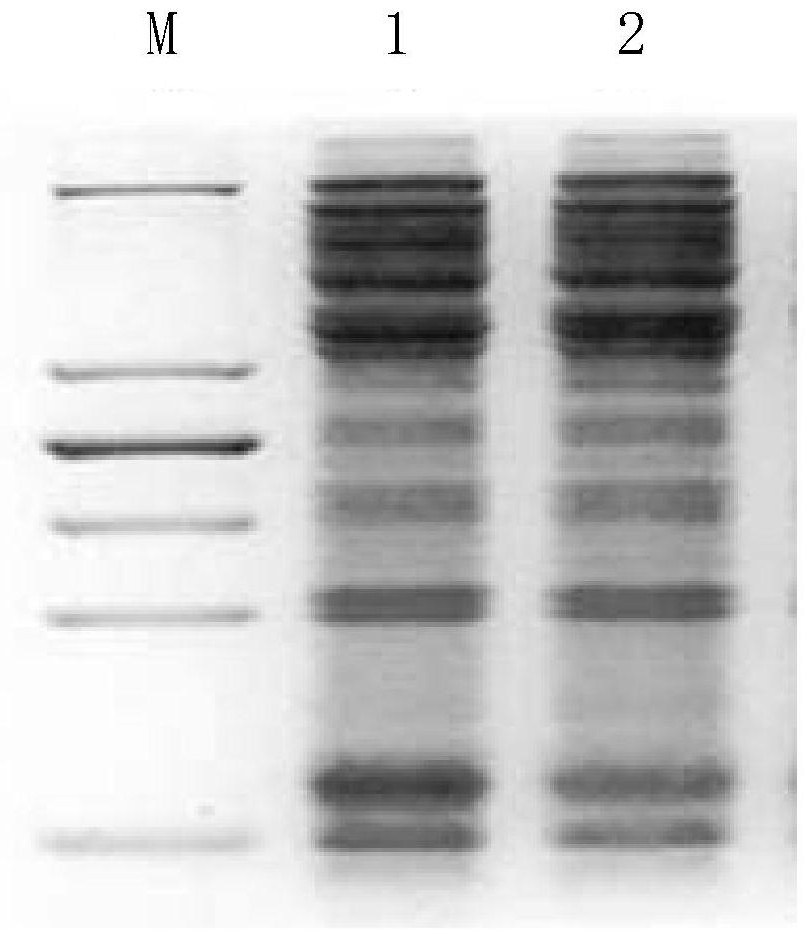
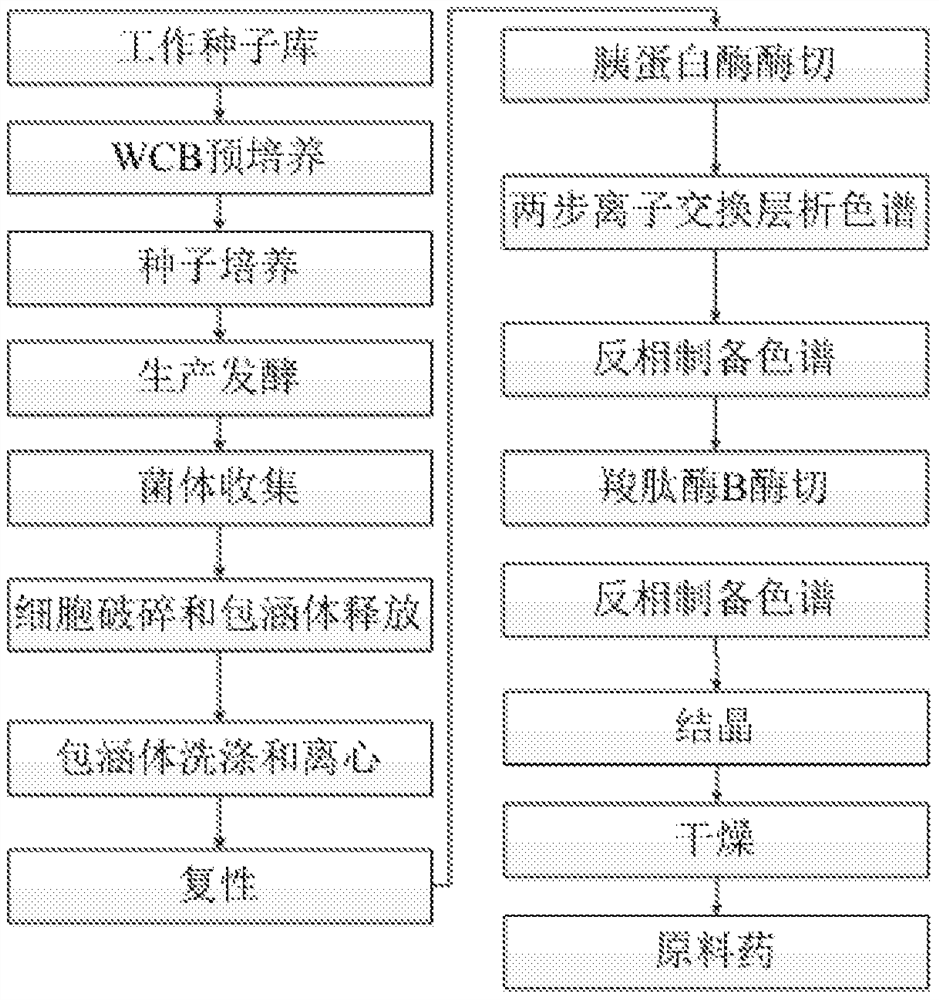
The invention provides a structural design of novel proinsulin aspart and a preparation method of insulin aspart. The main steps include designing the sequence of proinsulin aspart, constructing recombinant insulin aspart engineering bacteria, inducing the expression of insulin aspart fusion protein in the form of inclusion bodies through engineering bacteria, and then obtaining mature insulin aspart through denaturation, renaturation, enzyme digestion and separation and purification. Insulin aspart API. The invention avoids the dangerous and cumbersome step of cutting with hydrogen bromide by changing the sequence of the recombinant leader peptide and C-peptide; shortens the C-peptide to 1 amino acid to reduce the quality loss of enzyme digestion conversion.
Owner:AMPHASTAR NANJING PHARMA
Preparation method for yeast recombinant expression of insulin aspart
ActiveCN105087724BImprove digestion efficiencyLow miscut rateMicroorganism based processesInsulinsEnzyme digestionInsulin aspart
The invention discloses a preparation method for insulin aspart through recombinant expression by using yeast, and concretely relates to a preparation method for insulin aspart through recombinant expression by using pichia yeast. Concretely, the method comprises the following technological process: effectively secreting and expressing human aspart proinsulin, performing lysyl endopeptidase single enzyme digestion to obtain insulin aspart deleting B30, coupling with a threoninate, and performing deprotection, anti-phase purification and crystallization. The method is relatively suitable for industrialized preparation of recombinant insulin aspart.
Owner:CHONGQING PEG BIO BIOTECH CO LTD
A kind of preparation method of insulin aspart injection
ActiveCN108114270BThe process steps are simpleImprove stabilityPeptide/protein ingredientsMetabolism disorderMedicineInsulin aspart
The invention provides a preparation method of an insulin aspart injection, relating to the field of pharmaceutical preparations, and aims to provide the preparation method of the insulin aspart injection simple to operate, safe and reliable and stable in quality. The method comprises the following steps: a) providing an alkaline buffer solution; b) providing a mixed solution of a phenol mixture,an isoosmotic adjusting agent and a stabilizer; c) uniformly mixing the solution; d) adding insulin aspart and uniformly mixing the insulin aspart with the solution; or the method comprises the following steps: a') providing the alkaline buffer solution; b') adding insulin aspart to be mixed and dissolved; c') providing the mixed solution of the phenol mixture, the isoosmotic adjusting agent and the stabilizer; and d') uniformly mixing the solution.
Owner:NANJING HANXIN PHARMA TECH CO LTD
Insulin aspart injection and preparation method thereof
ActiveCN113384529AQuantitatively accurateQuality improvementPeptide/protein ingredientsMetabolism disorderInsulin injectionInsulin aspart
The invention provides a protamine sulfate insulin aspart crystal and a process method for preparing an insulin aspart premix preparation by using the protamine sulfate insulin aspart crystal, and particularly relates to a preparation method of an insulin aspart 50 injection and an insulin aspart 30 injection. The preparation obtained by the process method of the invention is regular in shape and uniform in size for the insulin crystal form, as well as has good stability. Most importantly, the process method provided by the invention is simple and stable, and very convenient for industrial application.
Owner:BEIJING HUIZHIHENG BIOTECHNOLOGY CO LTD
A kind of insulin aspart injection and preparation method thereof
ActiveCN113384529BQuantitatively accurateQuality improvementPeptide/protein ingredientsMetabolism disorderInsulin injectionInsulin aspart
The invention provides protamine sulfate insulin aspart crystallization and a process for preparing insulin aspart premixed preparations, and in particular relates to a preparation method of insulin aspart 50 injection and 30 injection. In the preparation obtained by the process provided by the invention, the crystal form of insulin has regular shape and uniform size, and the preparation has good stability. Most importantly, the process provided by the invention is simple and stable, and is very convenient for industrial application.
Owner:BEIJING HUIZHIHENG BIOTECHNOLOGY CO LTD +1
Preparation method of insulin aspart 30 suspension
PendingCN114306577ALow impurity contentImprove securityPeptide/protein ingredientsMetabolism disorderInsulin aspartDrugs preparations
The invention discloses a preparation method of an insulin aspart 30 suspension, and belongs to the field of pharmaceutical preparations. According to the invention, the stirring paddle with small shearing force is used for stirring the mixture composed of the insulin aspart prescription at a low speed instead of the existing standing crystallization method, such as an anchor paddle, a propeller, a frame paddle and the like, the stirring speed is 40-80rpm, the stirring time is 12-18h, and finally the quick-acting and long-acting insulin aspart 30 suspension is prepared. The insulin aspart suspension prepared in the invention has the advantages of small storage impurity content, uniform and stable particle size, strong storage stability, simple preparation process, short preparation time and high repeatability, and is suitable for industrial mass production.
Owner:NANJING HANXIN PHARMA TECH CO LTD
Inhalation preparation
PendingCN114432432AEasy to operateImprove controllabilityPowder deliveryDispersion deliveryDiabetes mellitusUse medication
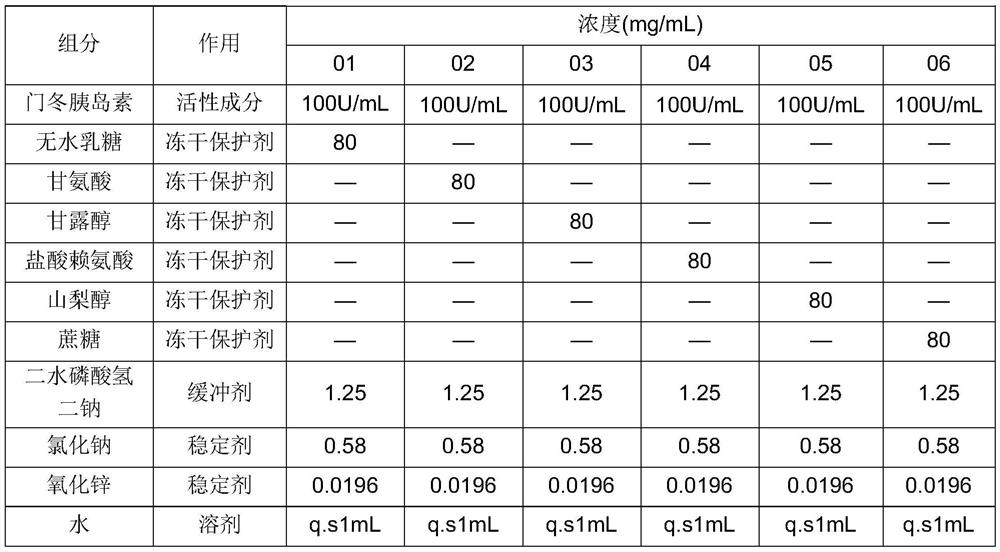
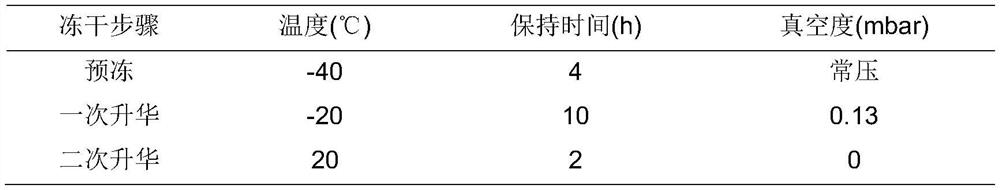
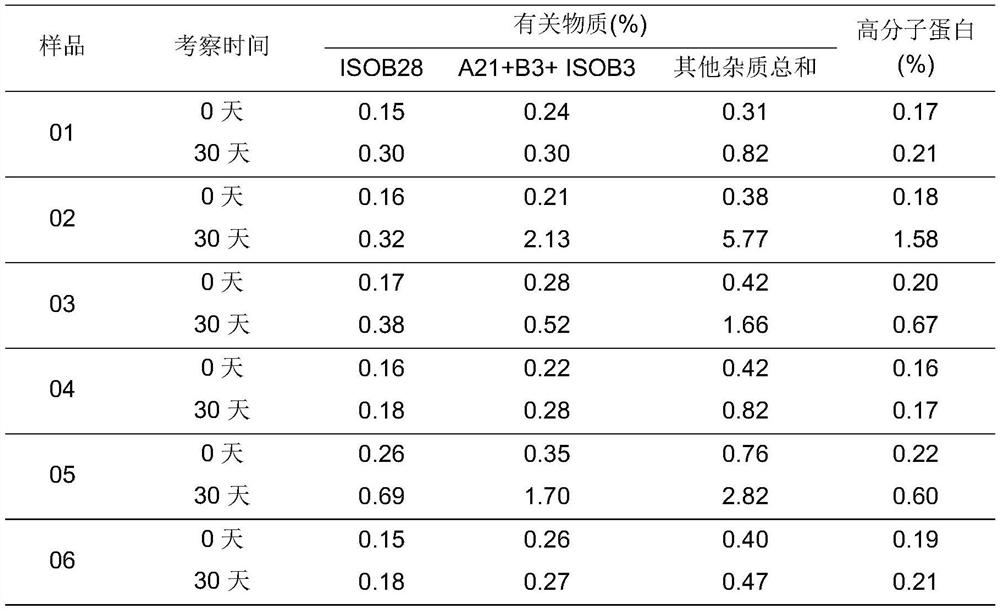
The invention relates to an insulin aspart inhalation preparation, and belongs to the technical field of pharmacy. The inhalation preparation is used for treating diabetes; the inhalation preparation comprises a freeze-drying protective agent, a buffering agent, a stabilizing agent, an isoosmotic adjusting agent, a bacteriostatic agent and the like. The inhalation preparation can improve the compliance of a patient, reduce the medication difficulty of the patient and reduce adverse reactions to the throat and the lung; the inhalation preparation is good in stability, the concentration of the medicine in the respiratory tract can be improved, the administration dosage is reduced, and the medication safety is improved.
Owner:SUNSHINE LAKE PHARM CO LTD
A method for efficiently removing residual organic solvents in insulin aspart
ActiveCN113074519BImprove product qualityDrying solid materials without heatDrying machines with local agitationOrganic solventInsulin aspart
The invention relates to the field of biomedicine, in particular to a method for efficiently removing solvent residues in the crystallization and drying process of insulin aspart. The insulin aspart crystallization drying process of the present invention adopts preliminary vacuuming to remove most of the organic solvents; then add water to keep the moisture and keep the pressure, during which temperature control and timely turning are required, so that the solvent crystals of insulin aspart are transformed into crystal water; Vacuumize, control the temperature and turn the material in time to further remove the difficult-to-remove residual organic solvents; repeat the moisturizing and pressure-holding and vacuuming steps to allow the wet solids to fully contact with water vapor, and ensure the stability of the sample on the basis of reducing solvent residues. The residual ethanol solvent in the kilogram-level insulin finished product prepared by the invention is less than 3000 ppm, and the product purity reaches more than 99%. It solves the problems of high solvent residue, unstable quality, high equipment requirements, high amplification cost and unsuitability for large-scale production existing in the prior art.
Owner:AMPHASTAR NANJING PHARMA
Kit for detecting insulin allergen
InactiveCN102038962AQuick checkEasy to operateIn-vivo testing preparationsBenzoic acidInsulin lispro
The invention relates to a detection kit, in particular to a kit for detecting insulin allergen. The kit for detecting the insulin allergen comprises recombinant human insulin and human insulin analog (insulin aspart, insulin lispro and insulin glargine), swine insulin, and various insulin additives including protamine, m-cresol, zinc, p-hydroxybenzoate, benzoic acid and isophane. Histamine and a salt solution are respectively used as positive and negative control solutions. Hence, the kit disclosed by the invention has the advantages of simplicity of operation, accuracy in results and low cost, can be used for detecting the insulin allergen quickly, is suitable for clinic popularization and application, and provides a very valuable and practical detection method for clinic and scientific researches.
Owner:HARBIN MEDICAL UNIVERSITY
A kind of insulin aspart injection and preparation method thereof
ActiveCN106177917BImprove the immunityIncreased sensitivityPeptide/protein ingredientsMetabolism disorderSide effectInsulin injection
The invention provides an insulin aspart injection and a preparation method thereof. The injection comprises insulin aspart, alpha-mannatide, a zinc salt and a bacteriostatic agent, wherein the concentration of insulin aspart in the injection ranges from 300 nmol / mL to 1,200 nmol / mL, and the concentration of alpha-mannatide in the injection ranges from 10 nmol / mL to 100 nmol / mL. The insulin aspart injection can improve insulin resistance and improve sensibility of target tissue to insulin, the medicine dose can be reduced to reach expected therapeutic indexes, the toxic and side effects such as hypoglycemia, edema and allergies cannot be caused, the possibility of occurrence of toxicity and medicine resistance is significantly decreased, and the treatment cost is reduced.
Owner:HEFEI TIANMAI BIOTECH DEV
Optimization and high-level expression of insulin aspart precursor gene
The invention discloses an optimized insulin aspart precursor gene sequence and its high-efficiency expression in yeast. The amino acid sequence encoded by the optimized insulin aspart precursor gene of the present invention is consistent with the original gene sequence, but the expression level in yeast is much higher than the original gene sequence. The expression results were detected by the shake flask fermentation test, and it was found that the protein expression of the insulin aspart precursor increased by 1.8 to 7.5 times after optimization; Increased by 10.8 times. The invention also discloses the modification method of the above gene sequence.
Owner:浙江海晟药业有限公司
Aspart proinsulin compositions and methods of producing aspart insulin analogs
Aspart modified proinsulin sequences that have a modified C-peptide amino acid and / or nucleic acid modification for producing aspart insulin analogs are provided. Highly efficient processes for preparing the aspart insulin analogs and improved preparations containing the aspart insulin analogs prepared according to the methods described herein are also provided.
Owner:AGILA BIOTECH PVT LTD
Preparation method of insulin aspart
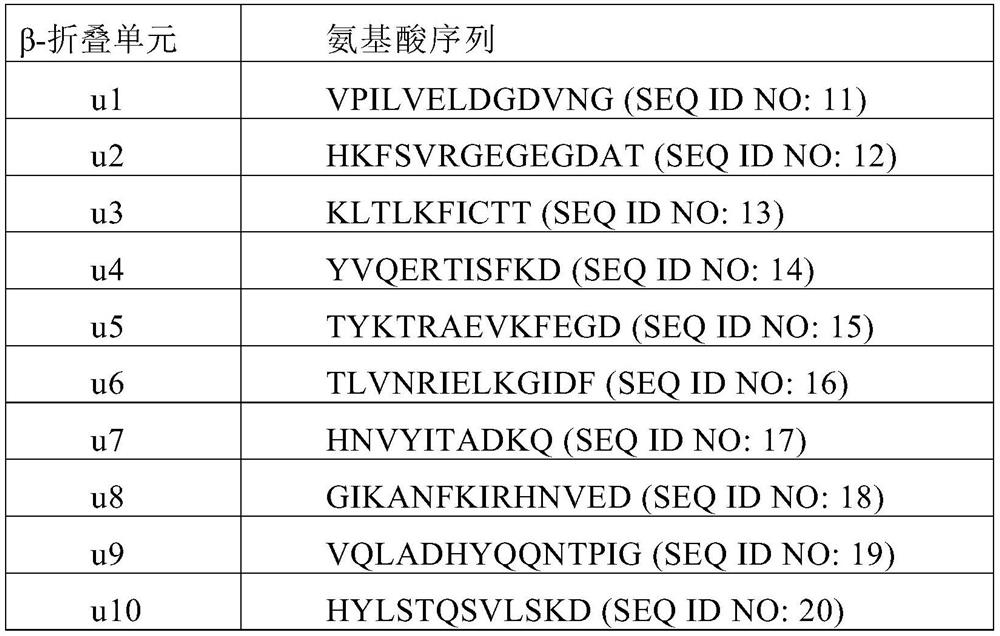
The invention provides an insulin aspart derivative and a preparation method thereof. Specifically, according to the method, insulin aspart fusion protein containing a green fluorescent protein folding unit is expressed in escherichia coli in a high-density mode, and enzyme digestion and purification are conducted on the fusion protein to prepare the insulin aspart. The method disclosed by the invention does not need to be carried out in a solid-phase organic system, process steps are reduced, environmental pollution is small, cost is lower, and the method is suitable for popularization.
Owner:NINGBO KUNPENG BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com