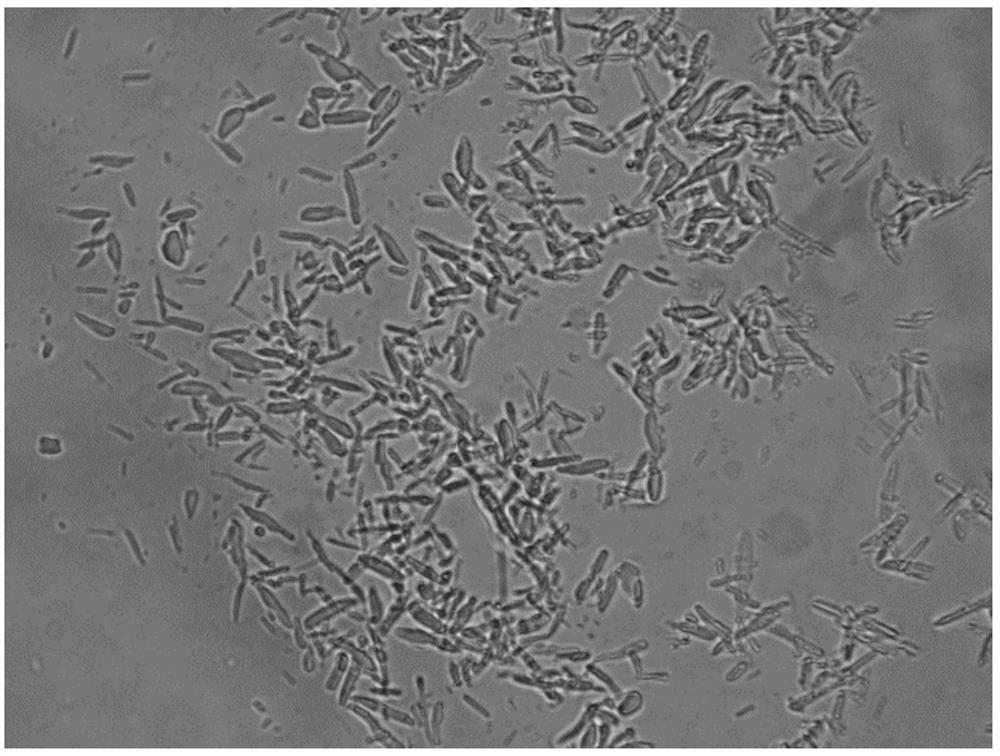
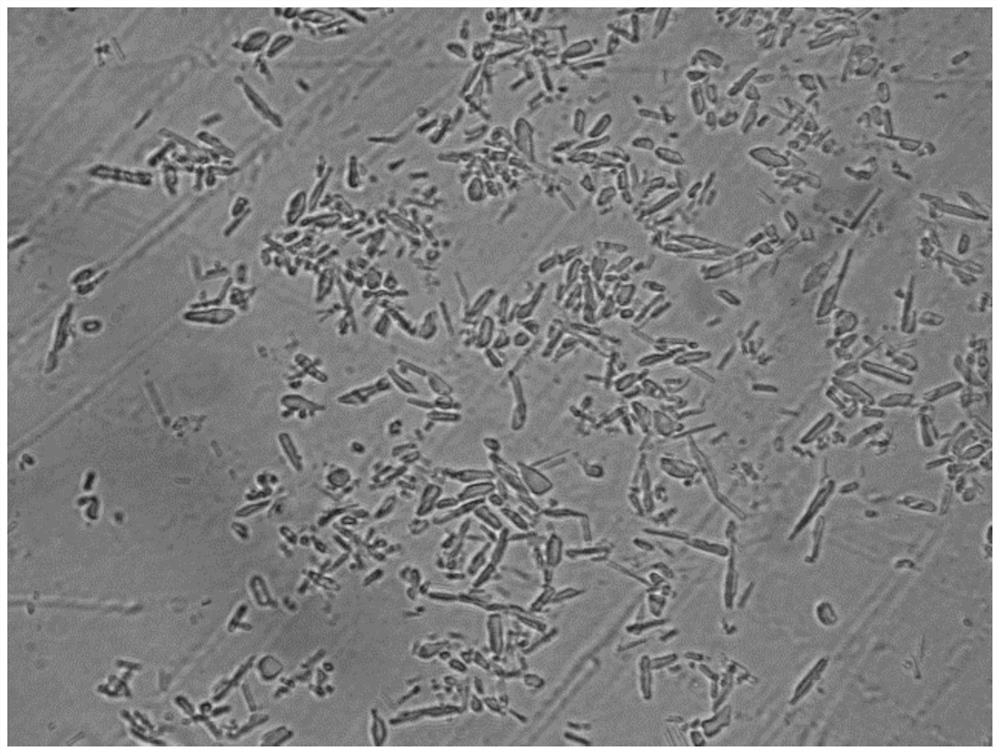
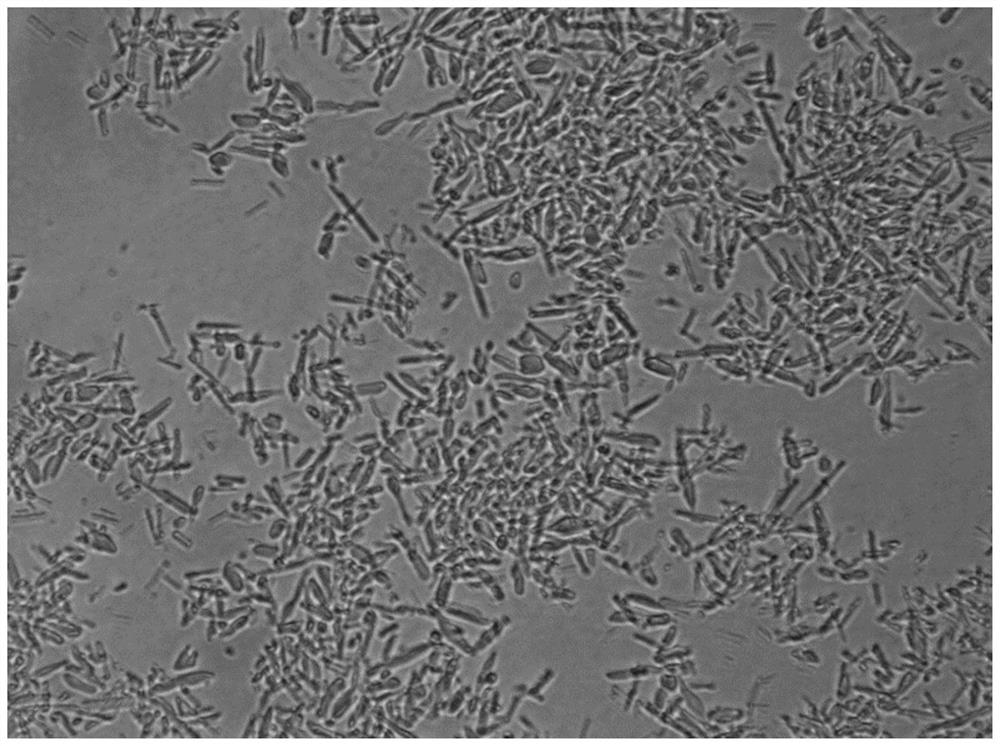
Preparation method of insulin aspart 30 suspension
A technology for insulin aspart and suspension, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, liquid delivery, etc. It can solve the problems of long standing crystallization time, preparation impurities, and high energy consumption in the process and other problems, to achieve the effect of low process energy consumption, uniform and stable particle size, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] 1. Prescription:
[0071] Table 1 The prescription of insulin aspart 30 suspension
[0072] prescription ingredients prescription content insulin aspart 35g glycerin 160g phenol 15g m-cresol 17.2g Zinc chloride 0.196g Sodium chloride 8.8g Disodium hydrogen phosphate dihydrate 12.5g protamine sulfate 3.2g
[0073] 2. Solution preparation:
[0074] (1): Take by weighing 12.5g prescription quantity disodium hydrogen phosphate dihydrate, 8.8g prescription quantity sodium chloride, 80g glycerin, 7.5g phenol, 17.2g prescription quantity m-cresol, dissolve with 4L water for injection and stir until clarification, Adjust the pH to 9.8, filter and sterilize the medicinal solution through a 0.22 μm filter membrane to obtain solution A, which is added to the liquid mixing tank;
[0075] (2): Weigh 35g prescription insulin aspart, 3.2g prescription protamine sulfate, add hydrochloric acid solution to dissolve, then a...
Embodiment 2
[0079] 1. the prescription is consistent with the prescription of embodiment 1.
[0080] 2. Solution preparation:
[0081] (i): Weigh 12.5g prescription quantity of disodium hydrogen phosphate dihydrate, 8.8g prescription quantity sodium chloride, 40g glycerin, 6g phenol, dissolve with 2.5L water for injection and stir until clear, adjust pH to 9.8, and pass through 0.22μm filter membrane to filter and sterilize to obtain solution A, which is added to the liquid mixing tank;
[0082] (ii): Take by weighing 35g prescription insulin aspart, 3.2g prescription protamine sulfate, after adding hydrochloric acid solution to dissolve, then add 0.196g prescription zinc chloride, 40g glycerol, 6g phenol, then use 2.5L water for injection After dissolving, adjust the pH to 2.5, filter and sterilize the drug solution through a 0.22 μm filter membrane to obtain solution B, which is added to the liquid mixing tank;
[0083] (iii): Stir and mix solution A and solution B evenly, adjust the ...
Embodiment 3
[0087] 1. the prescription is consistent with the prescription of embodiment 1.
[0088] 2. Solution preparation:
[0089] (1): Take by weighing 12.5g prescription quantity disodium hydrogen phosphate dihydrate, 8.8g prescription quantity sodium chloride, 80g glycerin, 7.5g phenol, 17.2g prescription quantity m-cresol, dissolve with 4L water for injection and stir until clarification, Adjust the pH to 9.5, filter and sterilize the medicinal liquid through a 0.22 μm filter membrane to obtain solution A, which is added to the liquid mixing tank;
[0090] (2): Weigh 35g prescription insulin aspart, 3.2g prescription protamine sulfate, add hydrochloric acid solution to dissolve, then add 0.196g prescription zinc chloride, 80g glycerin, 7.5g phenol, dissolve with 4L water for injection Finally, adjust the pH to 2.2, filter and sterilize the medicinal solution through a 0.22 μm filter membrane to obtain solution B, which is added to the liquid mixing tank;
[0091] (3): Stir and m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com