Patents
Literature
134 results about "High insulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nutritional system and methods for increasing longevity
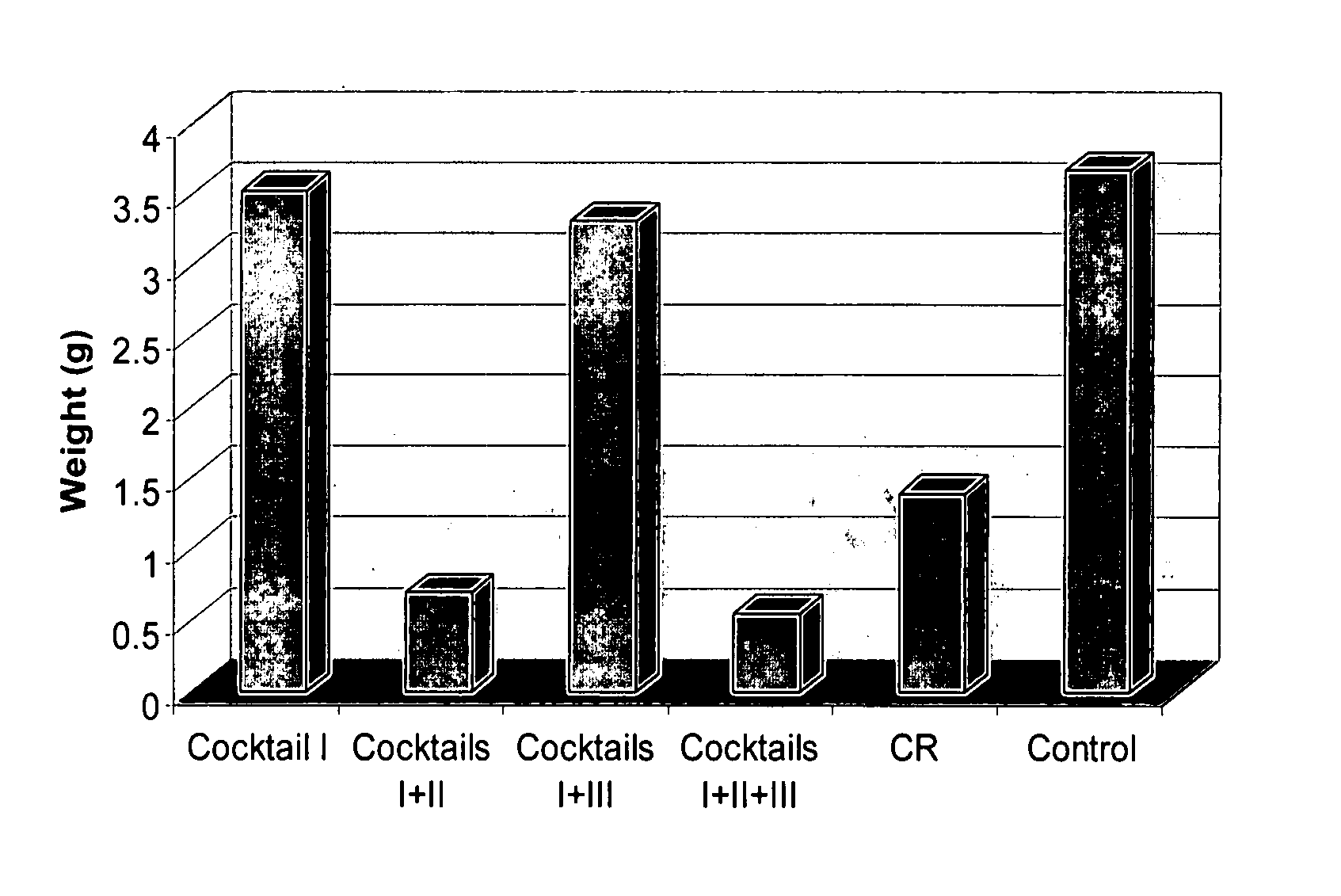
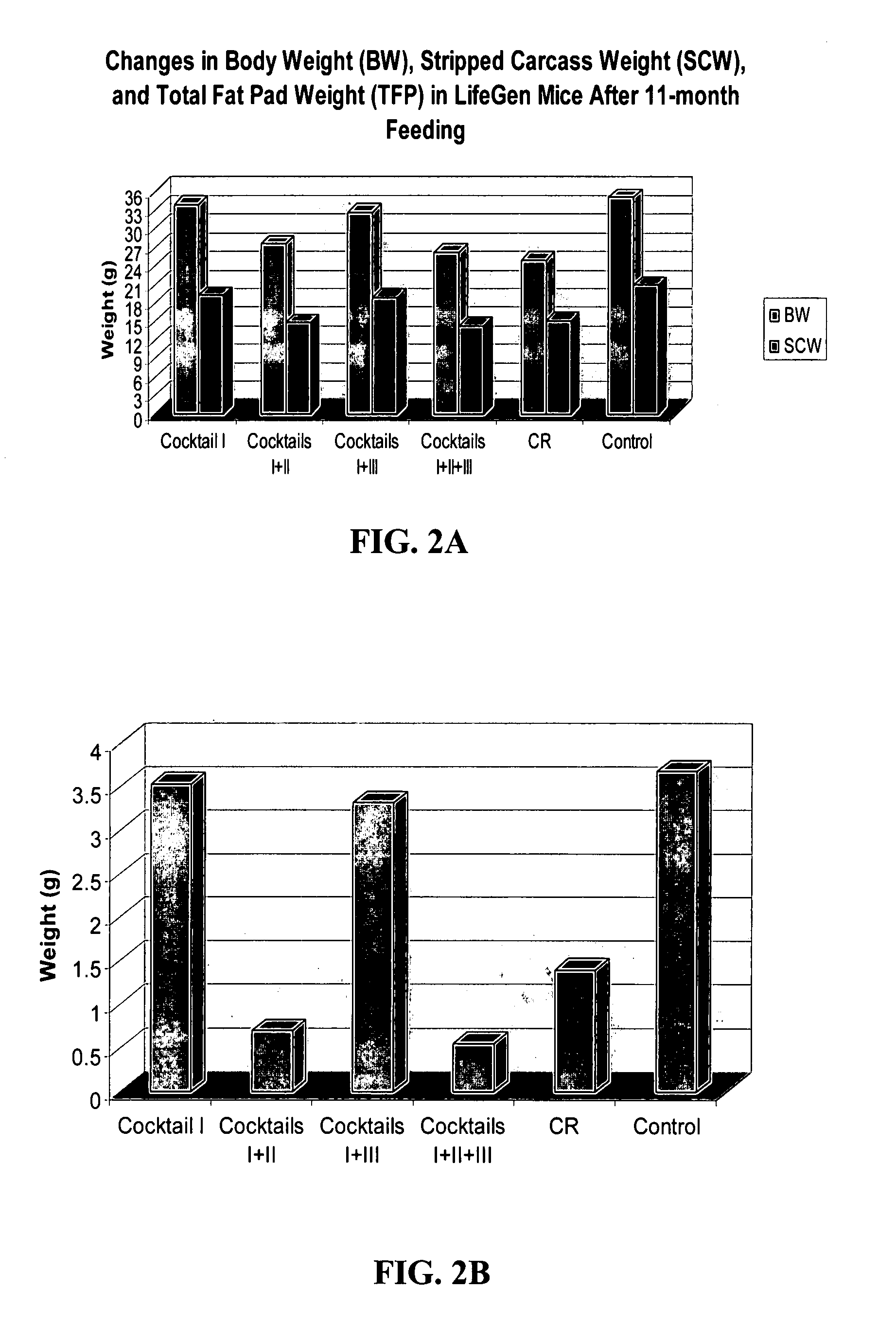
Disclosed herein are dietary formulations and methods to mimic the physiological, biochemical and gene expression effects of calorie restriction without altering dietary intake. The formulations include combinations of nutrients that have various intended functions in the body, falling into three or more of the following activities; (1) antioxidant activity; (2) inhibition of glycation damage; (3) reduction of body weight and fat; and (4) promotion of high insulin sensitivity and low blood insulin / glucose; and (5) anti-inflammatory activity.
Owner:NESTEC SA
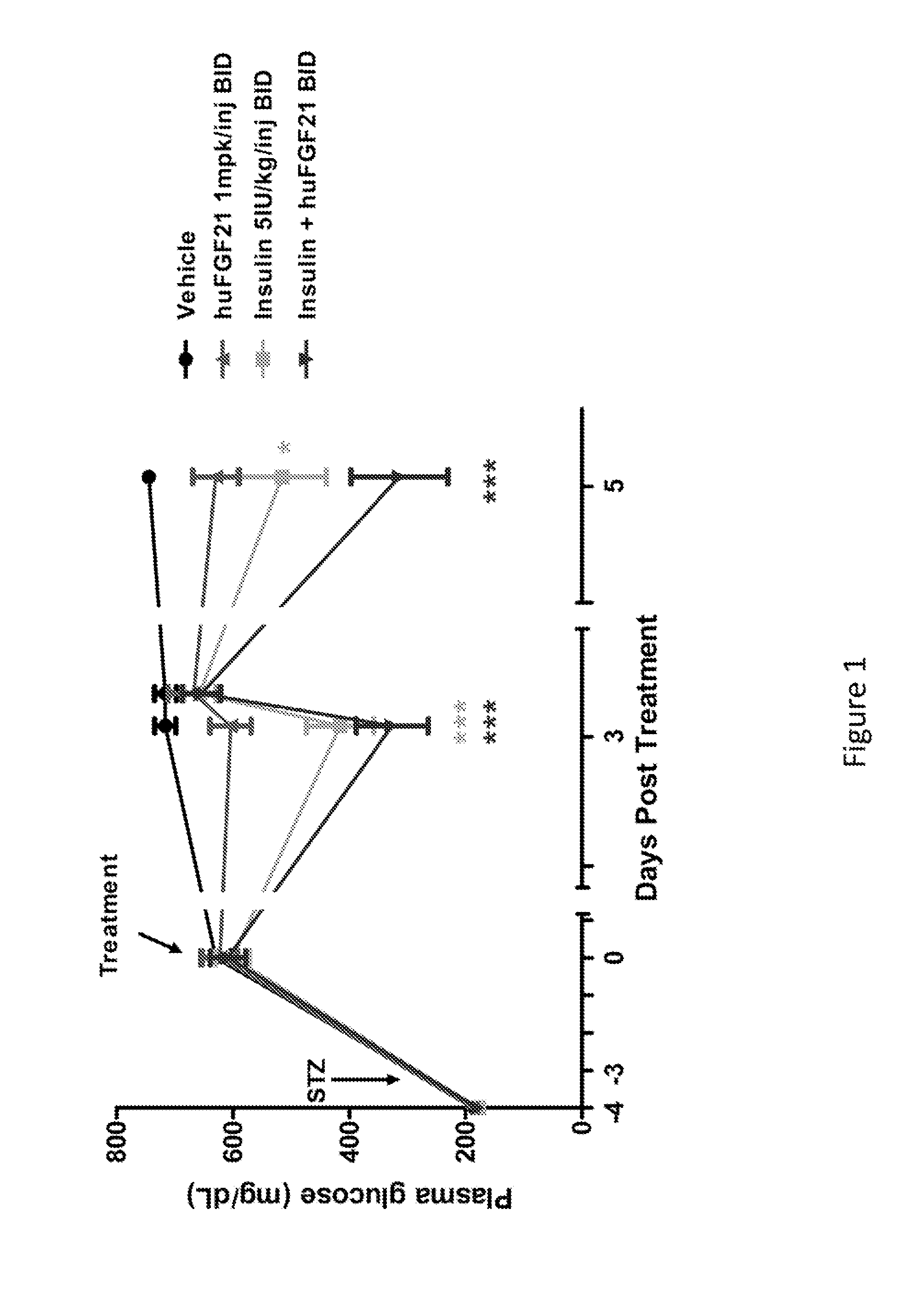
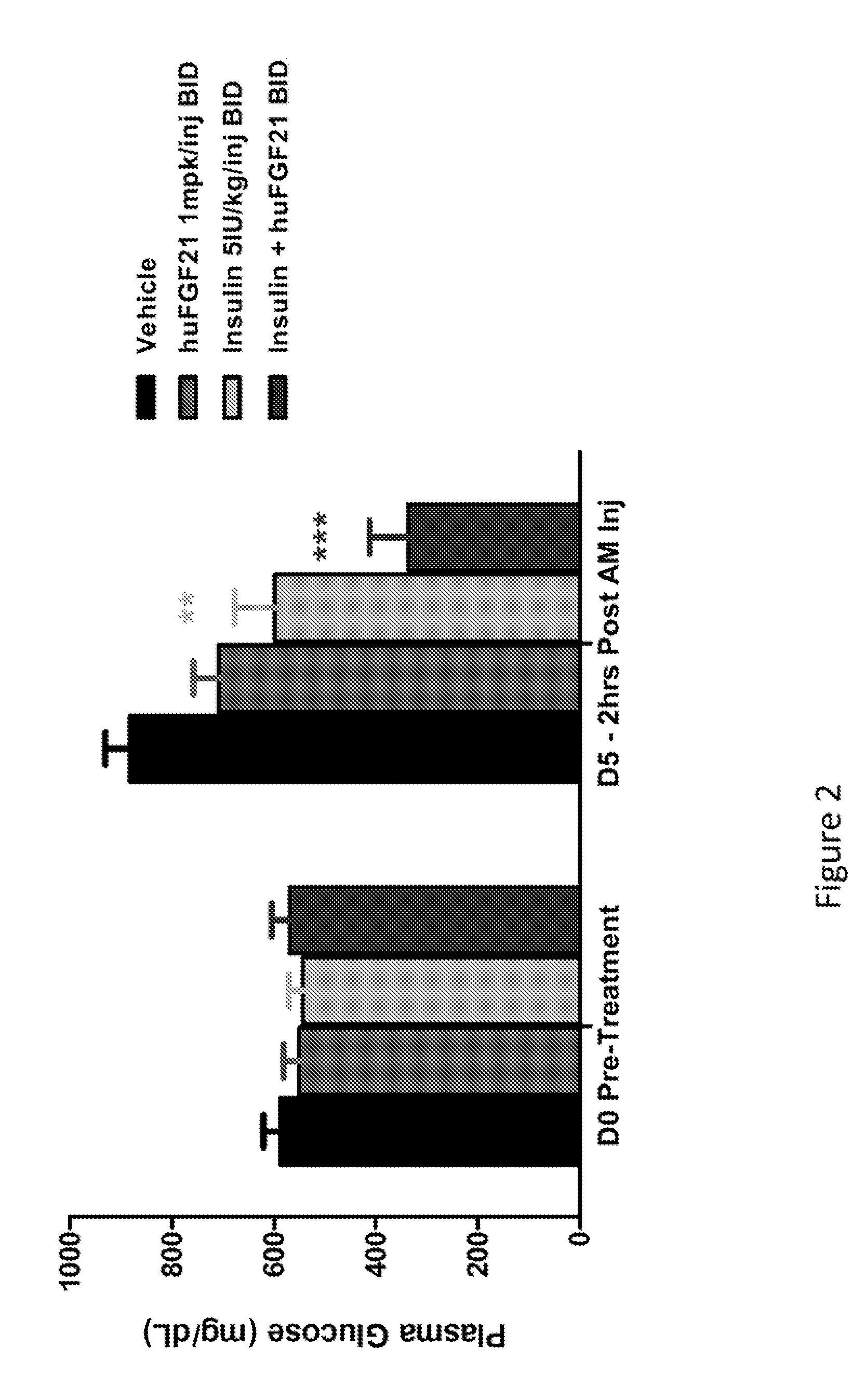
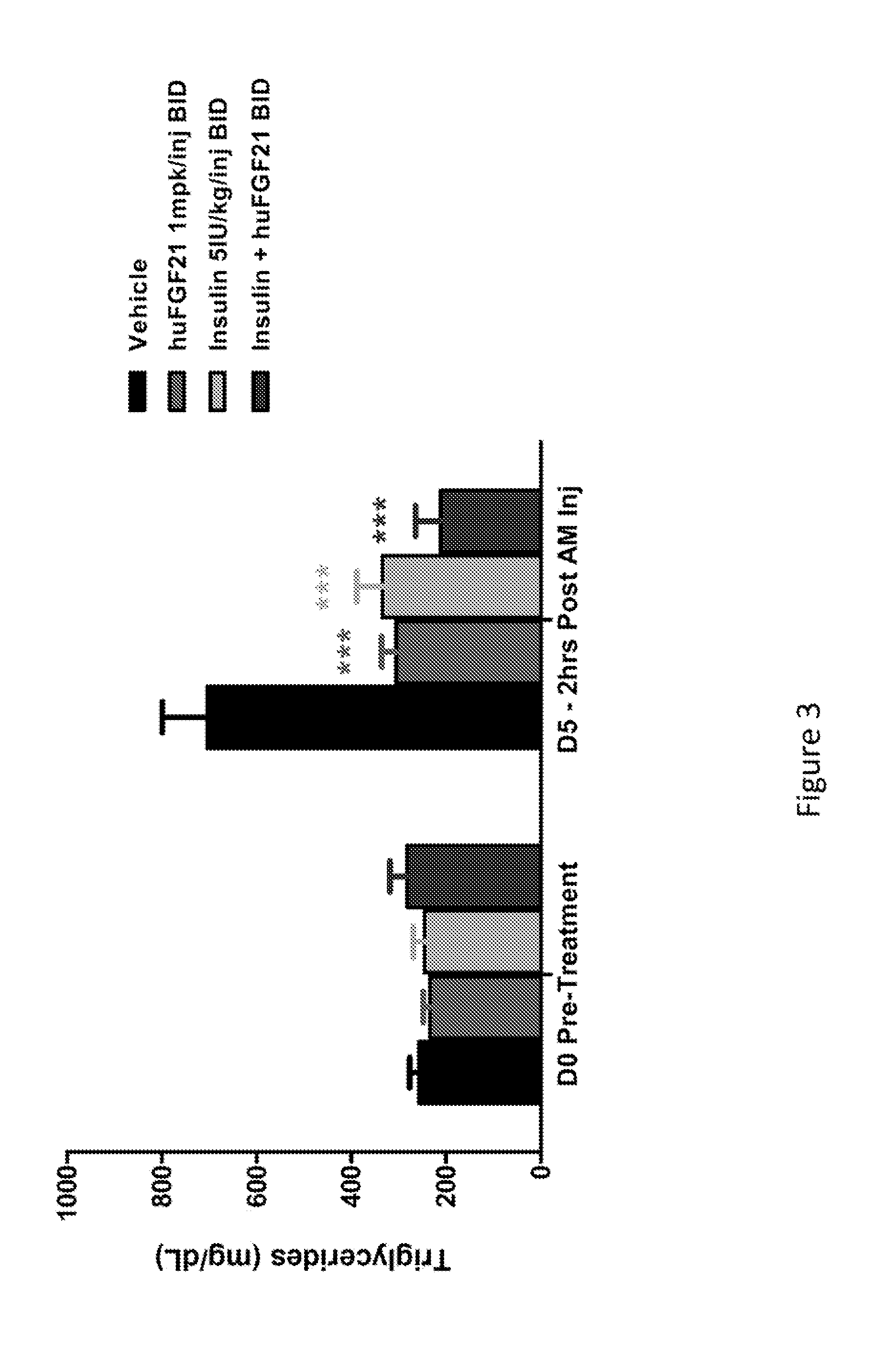
Method of Treating or Ameliorating Type 1 Diabetes Using FGF21
Methods of treating metabolic diseases and disorders using a FGF21 polypeptide are provided. In various embodiments the metabolic disease or disorder is type 1 diabetes, obesity, dyslipidemia, elevated glucose levels, elevated insulin levels, diabetic nephropathy, neuropathy, retinopathy, ischemic heart disease, peripheral vascular disease and cerebrovascular disease
Owner:AMGEN INC
Health-care noodle capable of regulating blood sugar and preparation method thereof
InactiveCN104187361AFull of nutritionSmooth tasteNatural extract food ingredientsFood ingredient functionsDandelionRice flour
Disclosed health-care noodle capable of regulating blood sugar is prepared from the following raw materials in parts by weight: 70-80 parts of whole wheat flour, 20-30 parts of tartary buckwheat powder, 10-15 parts of mung bean powder, 10-12 parts of coix lacryma-jobi powder, 10-12 parts of glutinous rice flour, 0.1-0.3 part of flower powder, 15-20 parts of purslane, 15-20 parts of chrysanthemum nankingense, 15-20 parts of dandelion, 15-20 parts of ilex kudingcha, 15-20 parts of mulberry leaf, 15-20 parts of corn silk, 15-20 parts of cyclocarya paliurus leaf, 10-12 parts of squab soup, and 0.2-0.4 part of a flour additive. The health-care noodle is abundant in nutrition and smooth in mouthfeel, does not stick to teeth and does not muddy soup when being boiled, and is capable of regulating blood sugar, improving blood circulation, improving insulin activity and accelerating glycometabolism after being frequently eated.
Owner:叶海峰
Etractive of fenugreek and its producing method
InactiveCN1480462AGood hypoglycemic effectInhibit aggregationOrganic active ingredientsMetabolism disorderDiabetes mellitusAlcohol
Owner:XINJIANG TEFENG PHARMA +1
Chitosan nanoparticle for improving absorption of orally delivered insulin by colon and preparation method of chitosan nanoparticle
ActiveCN105056212AHigh encapsulation efficiencyEnhanced interactionPeptide/protein ingredientsPharmaceutical non-active ingredientsChitosan nanoparticlesInsulin Cell
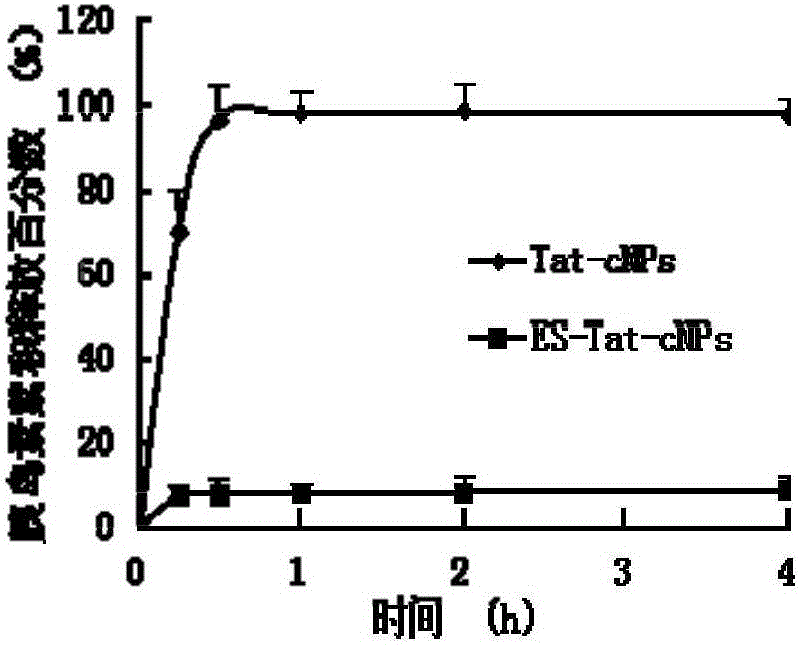
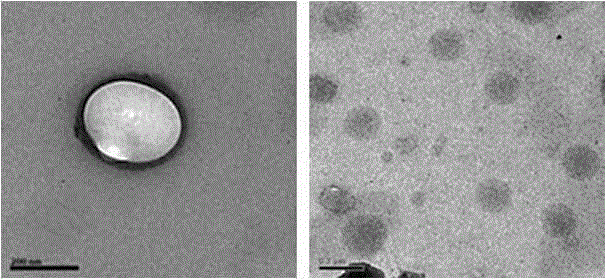
The invention relates to an enteric material coated chitosan nanoparticle which covers insulin and cell-penetrating peptide Tat and a preparation method of the chitosan nanoparticle. The chitosan nanoparticle is prepared from an enteric material, namely Eudragit S100, insulin, cell-penetrating peptide and chitosan. With the oral administration of the prepared enteric coated insulin chitosan nanoparticle, the amount of loaded contents released from the chitosan nanoparticle in the stomach and the upper part of the small intestine can be greatly reduced due to the protective effect of the enteric material, namely Eudragit S100. As the chitosan nanoparticle arrives at the lower end of the digestive tract, the enteric coating material on the surface of the chitosan nanoparticle begins to dissolve and the chitosan nanoparticle becomes exposed with the increase of pH value; the chitosan nanoparticle is damaged under a weakly alkaline condition, so that the cell-penetrating peptide and the insulin therein are released. The cell-penetrating peptide is capable of prompting the absorption of colonic epithelial cells to the insulin so as to improve the bioavailability of the insulin through oral administration.
Owner:江西省药物研究所
Insulin oral nano-preparation and preparation method thereof
InactiveCN104353062AExtended stayImprove oral bioavailabilityPowder deliveryPeptide/protein ingredientsBiocompatibilityBioavailability
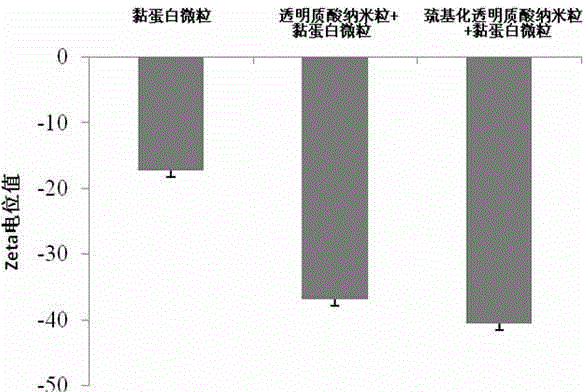
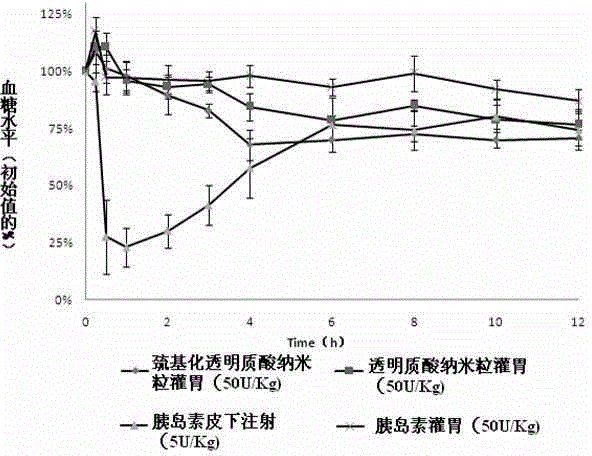
The invention discloses an insulin oral nano-preparation and a preparation method thereof. Polymer sulfhydrylated hyaluronic acid with biocompatibility and bio-adhesion is adopted as a carrier to prepare an insulin-entrapped nano-suspension, and a lyophilized preparation is finally prepared. According to the method, the bio-adhesive material sulfhydrylated hyaluronic acid is firstly used as the carrier to prepare sulfhydrylated hyaluronic acid nano-particles, the insulin oral bioavailability can be improved by the preparation, and the preparation has the characteristic of excellent stability, is easy to store and transport, is convenient for patient using, and is a preparation with wide development prospect.
Owner:FUZHOU GENERAL HOSPITAL OF NANJING MILITARY COMMAND P L A
Cyclocarya paliurus preparation and preparation method thereof
InactiveCN104940282ARegulate blood sugarImprove immunityMetabolism disorderGranular deliveryDyslipidemiaPhysiology
The invention provides a cyclocarya paliurus preparation and a preparation method thereof. The preparation can be easily and quantificationally administrated and the mouth feel of the preparation is acceptable to people who do not like to drink cyclocarya paliurus tea. The cyclocarya paliurus preparation is suitable for fat people of over 40 years in age, people having the family history of diabetes, people having hyperglycemia at one time, pregnant women of over 30 years in age and having fetal macrosomia delivery at one time, people having hyperglycemia accompanied with dysarteriotony and dyslipidemia, people having two symptoms out of hypeluricemia, hyperviscosily and hyperinsulinism, people who do not participate in manual labour all the year round, and people of valetudinarianism and hypoimmunity.
Owner:BAIHUA YIHUA GRP
Marine oligosaccharide compound with type II diabetes resisting activity
ActiveCN101649004AGood anti-type II diabetes activityImprove securityOrganic active ingredientsSugar derivativesPolymannuronic acidCarboxyl radical
The invention relates to a marine oligosaccharide compound with type II diabetes resisting activity, which is a compound which is formed by following steps: taking D-polymannose aldehydic oligosaccharide which comes from sea and contains carboxyl in each oligosaccharide ring, reacting with dilute alkali and chromium salt solution and introducing trivalent chromium ions which is closely relative tothe generation and the development of diabetes into oligosaccharide molecules. A pharmacological experiment proves that the product has remarkable effect on accelerating insulin secretion, is not influenced by amylin and has effects on lightening glucose load, improving blood-lipoid metabolism, insulin sensitivity and certain kidney protection and lightening pancreatic injury for type II diabetesrats and mouse. The product of the invention comes from marine natural species, has the advantages of good security, unique structure, low molecular weight, high chromium binding ratio, good oral absorption effect, and the like, can play a hypoglycemic role in a plurality of links and has favorable market application prospect on the aspect of preventing and treating type II diabetes.
Owner:OCEAN UNIV OF CHINA
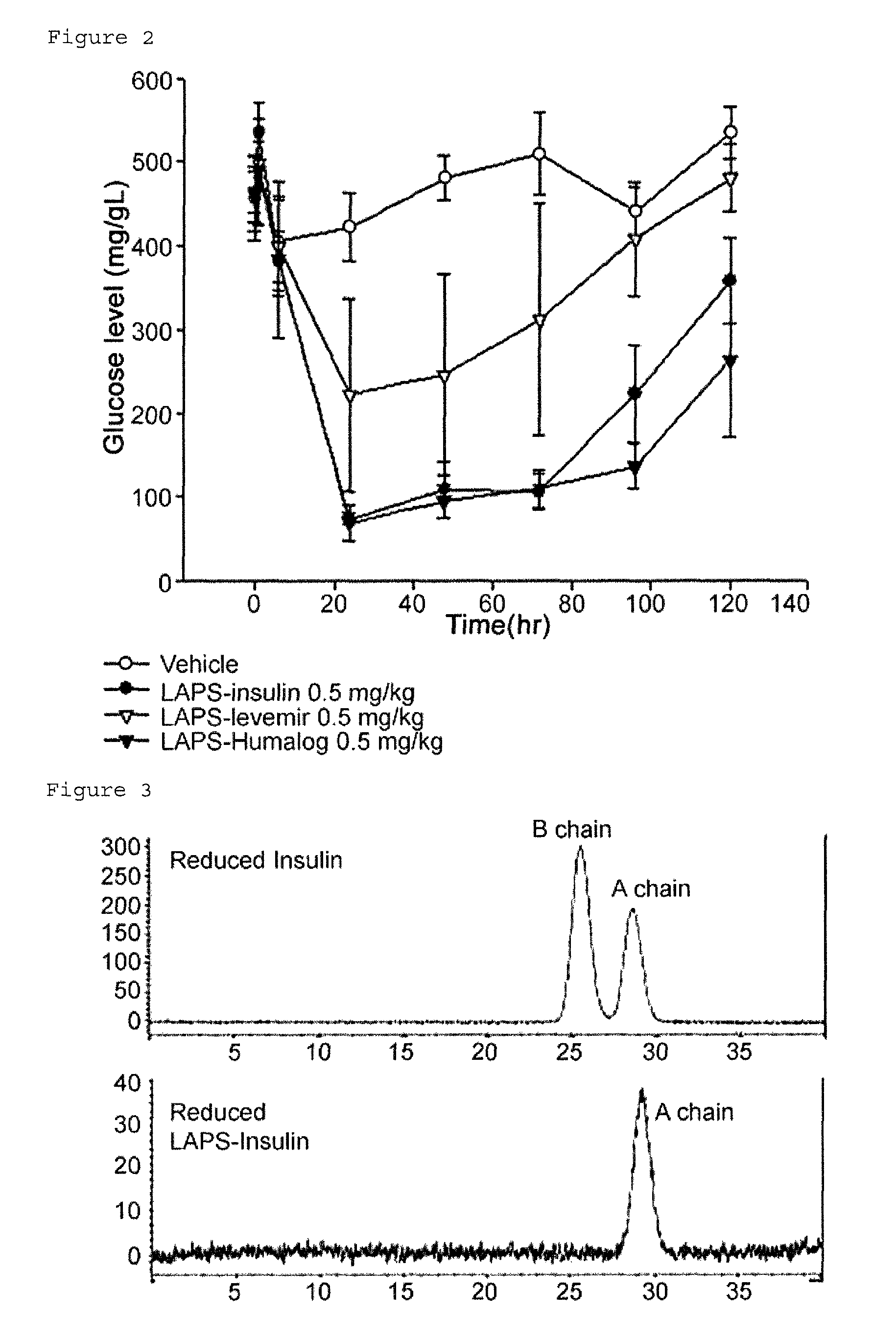
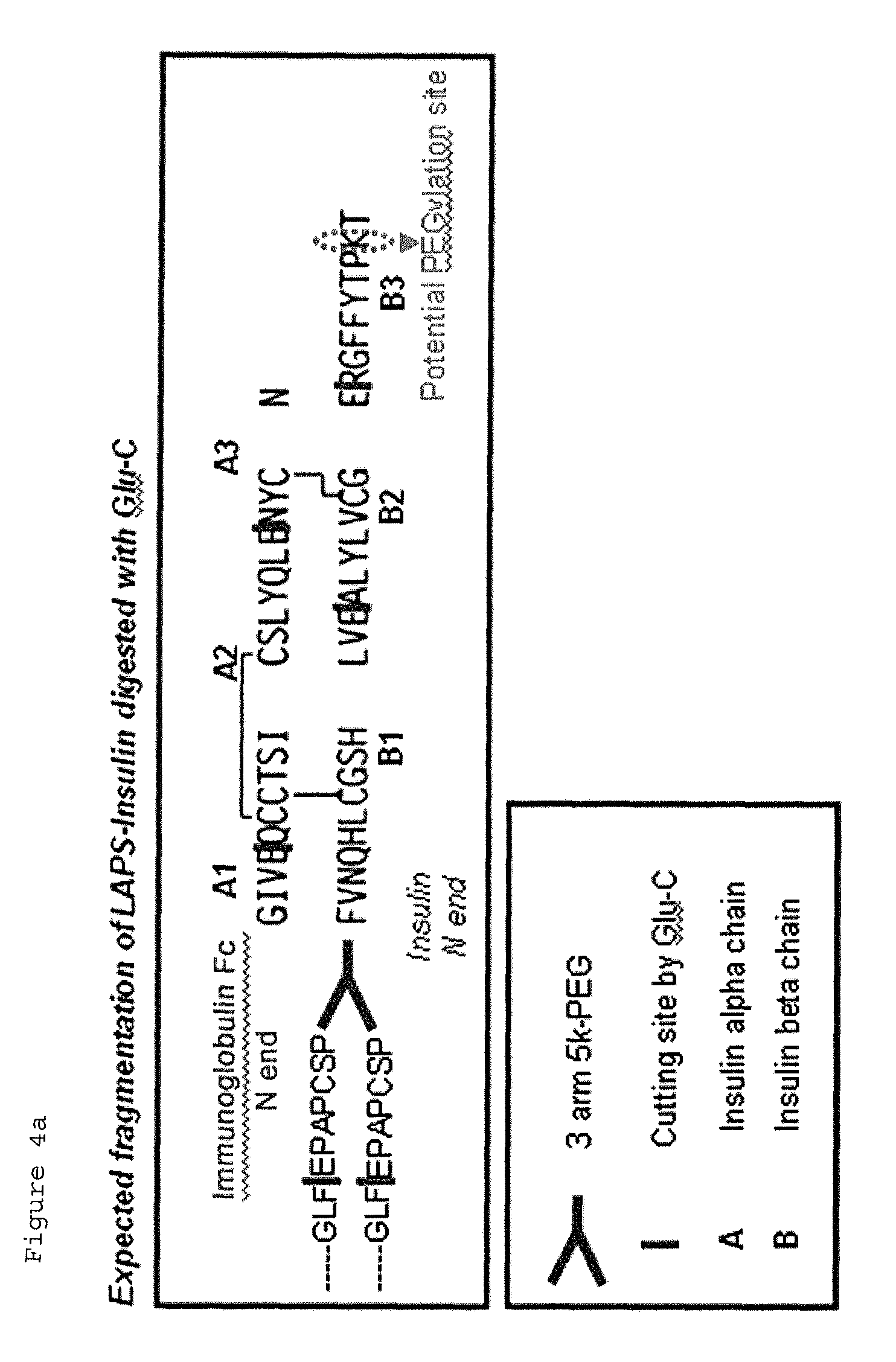
Insulin conjugate using an immunoglobulin fragment
ActiveUS9492507B2Extended half-lifeImprove Medication AdherencePeptide/protein ingredientsMetabolism disorderHalf-lifeIn vivo
Owner:HANMI SCI CO LTD
Treatment of hyperinsulinemic hypoglycemia with exendin-4 derivatives
In one aspect, methods of treating hyperinsulinemic hypoglycemia comprising administration of an effective amount of a derivative of an exendin-4 peptide are provided. In some embodiments, the method comprises subcutaneously administering to a patient having hyperinsulinemic hypoglycemia a therapeutically effective amount of exendin(5-39).
Owner:EIGER BIOPHARMLS
Application of diosmetin in preparation of medicine used for treating type 2 diabetes mellitus
InactiveCN106822087AExpand the scope of useImprove the level ofOrganic active ingredientsMetabolism disorderLevel insulinGlucose lowering
The invention relates to application of diosmetin in preparation of a medicine used for treating type 2 diabetes mellitus. Researches show that diosmetin can obviously improve glycometabolism of the type 2 diabetes mellitus, can improve insulin level and lower blood lipid level, can be used for preparing a blood-glucose-lowering medicine and provides a new medicine for treatment of the type 2 diabetes mellitus, thereby having significance on the treatment of diabetes mellitus.
Owner:SOUTHWEST UNIVERSITY
Application of dioscin in preparing medicament for preventing and treating diabetes mellitus
ActiveCN102552299AImprove the immunityRegulating metabolic disordersOrganic active ingredientsMetabolism disorderA lipoproteinPancreatic hormone
The invention discloses application of dioscin in preparing a medicament for preventing and treating diabetes mellitus. According to the invention, the effects of dioscin in reducing blood-sugar content of various hyperglycemia models (I-type diabetes mellitus, II-type diabetes mellitus and acute hyperglycemia caused by adrenaline) and increasing the glycogen content of the hyperglycemia models are discovered, and the effects of the dioscin in improving the cell insulin resistance caused by high insulin, reducing contents of total cholesterol, triglyceride and low-density lipoprotein, increasing content of high-density lipoprotein and regulating fat metabolism disorder are simultaneously discovered, so that the application of the dioscin in preparing the medicament for preventing and treating the diabetes mellitus is invented. Various preparations prepared from the dioscin serving as a raw material according to pharmaceutically related requirement and clinical requirement can be applied clinically, and have the advantages of safety (no toxic or side effect), convenience, economy and the like.
Owner:DALIAN MEDICAL UNIVERSITY
Buckwheat-pine pollen composite functional Kbac drink and preparation method thereof
InactiveCN103525624AAnti-fatigueClear colorMicroorganism based processesAlcoholic beverage preparationBiotechnologyPolygonum fagopyrum
The invention discloses a buckwheat-pine pollen composite functional Kbac drink and a preparation method thereof. The drink is prepared by taking buckwheat and pinus massoniana pollen as the main raw materials, and has the content of flavonoid over 0.5% and the content of pollen polysaccharide more than 0.2g / L. The preparation method comprises the following steps: (1) preparing buckwheat bud juice; (2) preparing pine pollen extraction liquid; (3) domesticating strain; (4) determining the formula of the Kbac drink; (5) preparing the Kbac drink. The composite functional Kbac drink disclosed by the invention is favorable for protecting heart and blood vessels, improving microcirculation, protecting liver, reducing blood glucose and blood lipid and enhancing the sensitivity of insulin receptor; meanwhile, the composite functional Kbac drink has the functions of resisting fatigue and adjusting intestinal and immune functions as well as composite nutrient and healthcare function; moreover, with clear color, strong taste and mellow mouthfeel, the composite functional Kbac drink can increase the types of drink, adapts to the tastes of different people and can promote the physical health of people.
Owner:GUIZHOU UNIV
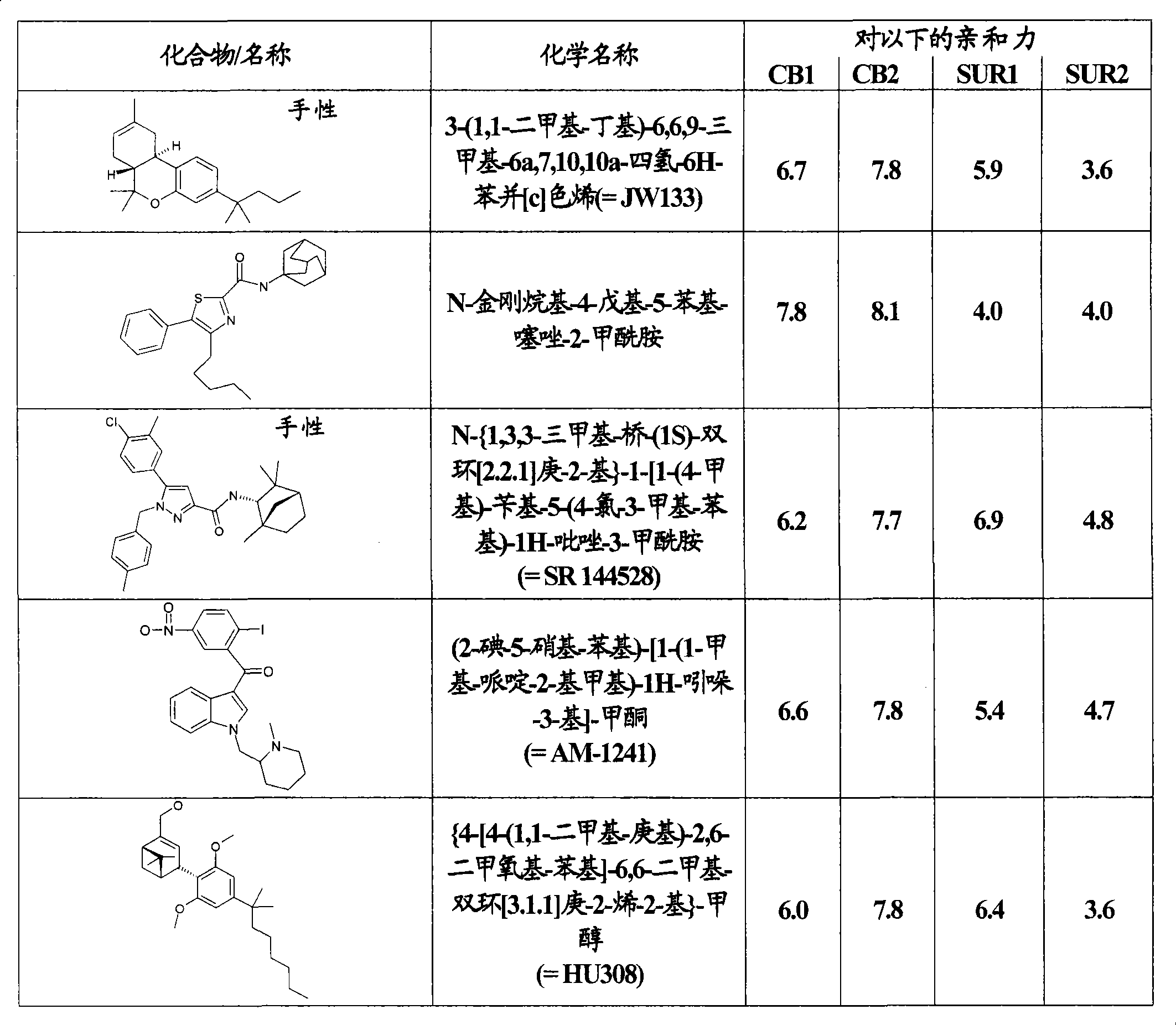
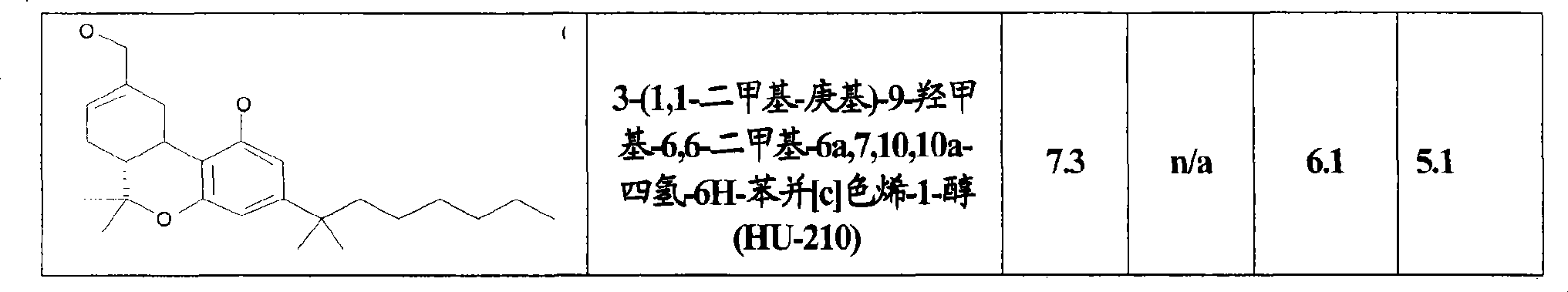
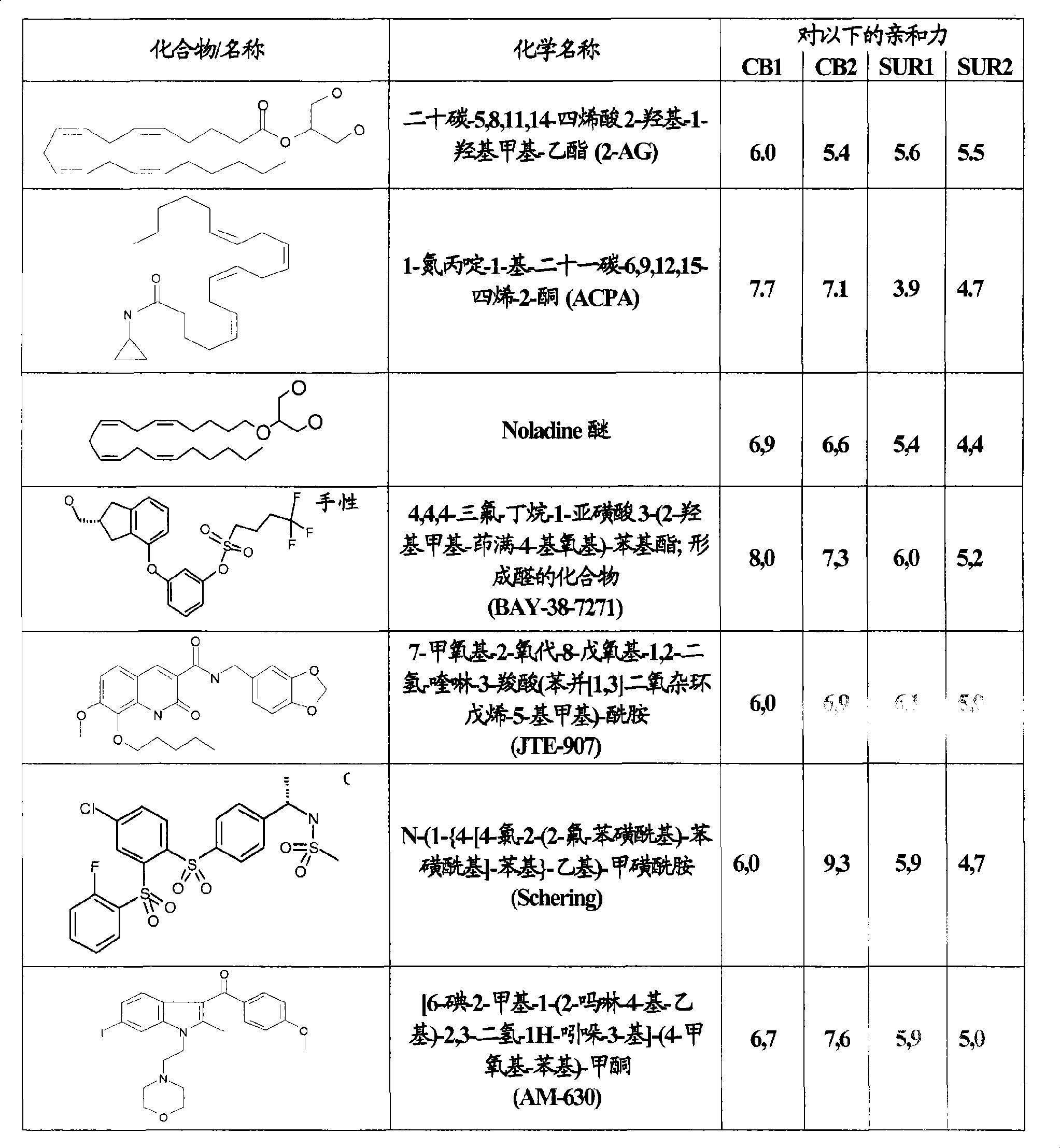
Use of CBx cannabinoid receptor modulators as potassium channel modulators
InactiveCN101431994AReduce processReduce seizuresNervous disorderMetabolism disorderAppetite regulationEpilepsy
The invention is directed to the use of at least one CBx modulator wherein the CBx modulator is selected from the group consisting of CB1 agonists; CB2 agonists; CB2 partial agonists; CB2 antagonists; CB2 inverse agonists; and dually acting compounds which are both a CB1 agonist and a CB2 agonist; and mixtures thereof, as KATP channel modulator for the prophylaxis, treatment, delayed progression, delayed onset and / or inhibition of a variety of disease conditions including obesity, diabetes mellitus, metabolic syndrome, syndrome X, insulinoma, familial hyperinsulemic hypoglycemia, male pattern baldness, detrusor hyperreactivity, asthma, neuroprotection, epilepsy, analgesia, cardioprotection, angina, cardioplegia, arrhythmia, coronary spasm, peripheral vascular disease, cerebral vasospasm, appetite regulation, neurodegeneration, pain - including neuropathic pain and chronic pain - and impotence in mammals and humans. The invention further relates to methods of treating, preventing, delaying progression of, delaying onset of and / or inhibiting a variety of disease conditions including obesity, diabetes mellitus, metabolic syndrome, syndrome X, insulinoma, familial hyperinsulemic hypoglycemia, male pattern baldness, detrusor hyperreactivity, asthma, neuroprotection, epilepsy, analgesia, cardioprotection, angina, cardioplegia, arrhythmia, coronary spasm, peripheral vascular disease, cerebral vasospasm, appetite regulation, neurodegeneration, pain - including neuropathic pain and chronic pain - and impotence in mammals and humans comprising administering to a subject in need thereof an effective amount of at least one CBx modulator having KATP channel modulating properties.
Owner:SOLVAY PHARMA GMBH
Insulin spray for oral cavity and its prepn process
InactiveCN1335182AIncrease concentrationIncrease contact areaPowder deliveryPeptide/protein ingredientsPhosphateOil phase
The present invention relates to medicine and its production and aims at raising biological utilization of oral absorption and strengthening the stability of the preparation. The insulin spray for oral cavity contains insulin 10000-70000 U, soybean lecithin 5-50 g and propylene glycol 25-80 g as well as borneol 1.2-10g, absolute ethyl alcohol 1-15 ml and phenol 2-5 g, the rest is pH 6.8-7.8 buffering phosphate solution, in each 1000 ml of microemulsion. The preparation process includes mixing soybean lecithin with propylene glycol, borneol and ethanol solution, addition of phenol containing buffering phosphate solution, supersonic treatment to obtain emulsified oil phase, the dissolution of insulin in the buffering solution, further mixing and supersonic treatment.
Owner:HUAZHONG UNIV OF SCI & TECH +1
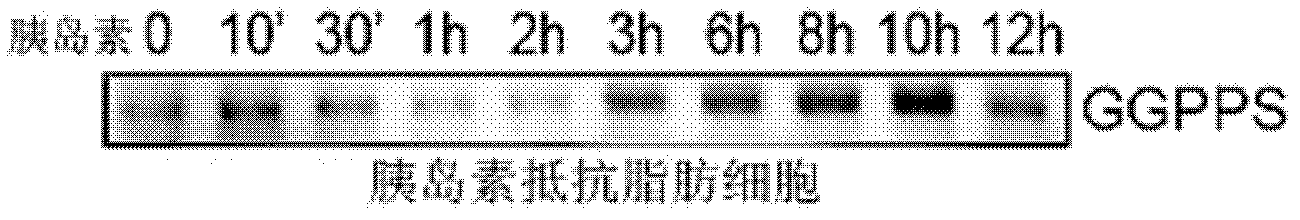
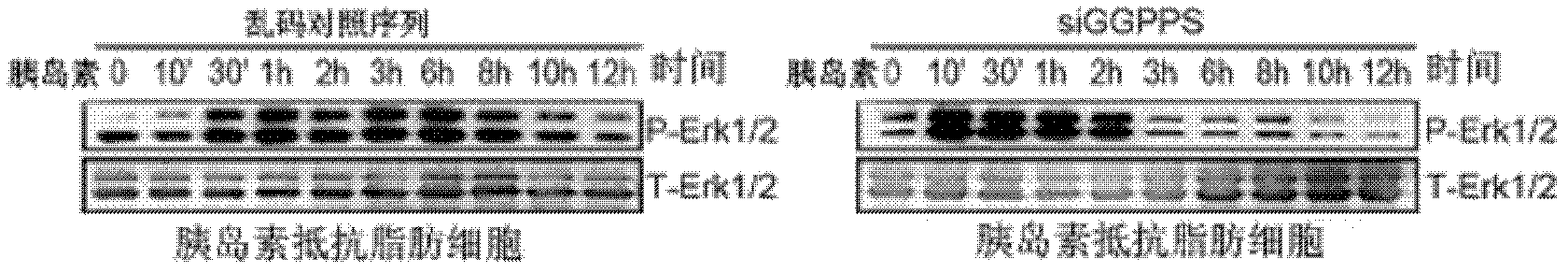
Application of geranylgeranyl diphosphate synthase (GGPPS) antagonist in preparation of medicament for treating II-type diabetes mellitus
InactiveCN102526763AIncreased sensitivityImprove the quality of lifeMetabolism disorderGenetic material ingredientsHigh insulinPancreatic hormone
The invention discloses application of geranylgeranyl diphosphate synthase (GGPPS) for treating II-type diabetes mellitus, and particularly provides application of a GGPPS antagonist in preparation of medicament for treating the II-type diabetes mellitus. The GGPPS gene and protein antagonist capable of being used for preparing sensitization medicaments is firstly disclosed. Furthermore, the GGPPS antagonist facilitates improvement on sensibility of insulin target organization on insulin, enhances treatment effects of the insulin, improves life quality of II-type diabetics, and has potential and good application prospect in the field of the II-type diabetes mellitus treatment.
Owner:NANJING UNIV
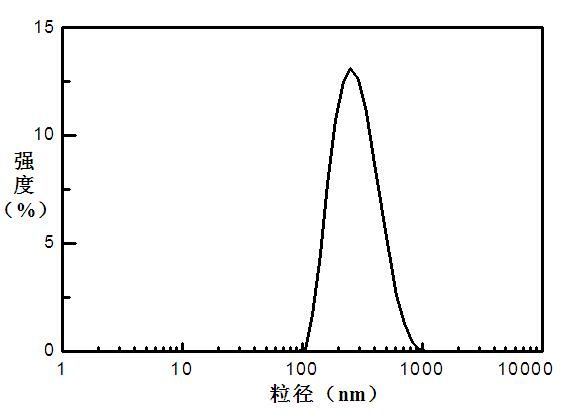
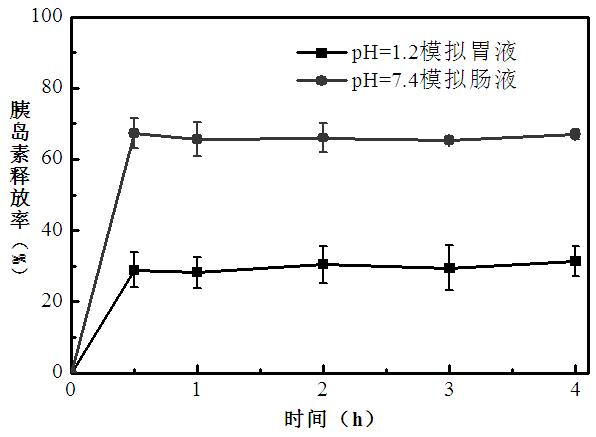
A kind of oral pegylated insulin pH-sensitive nanoparticles and preparation method thereof
InactiveCN102293748AInhibition releaseQuick releasePowder deliveryPeptide/protein ingredientsPEGylated insulinFreeze-drying
The invention discloses an oral PEGylated insulin pH-sensitive naonparticle and a preparation method thereof. The naonparticle is prepared from PEGylated insulin, pH-sensitive polymer, carrier, additives and stabilizer. The preparation method of the naonparticle comprises the following steps: preparing a PEGylated insulin / pH-sensitive polymer / carrier W / O primary emulsion, dispersing the W / O primary emulsion in a stabilizer solution to form a W / O / W multiple emulsion, obtaining a crude product after the solvent volatilizes, purifying the crude product, and carrying out freeze-drying to obtain the naonparticle. The PEGylated insulin pH-sensitive naonparticle disclosed by the invention has the advantages of uniform dispersion and high medicine enveloping rate; the insulin modified by polyethylene glycol can reduce the aggregation of insulin molecules, prolong the half life in vivo, and prolong the time of blood sugar reduction effect; and the release rate of the insulin in the gastric acid environment is reduced, the release rate of the insulin in the intestinal tract environment is enhanced since the naonparticle swells, and thus, the oral PEGylated insulin pH-sensitive naonparticle has an oral application value.
Owner:SOUTH CHINA UNIV OF TECH
Chinese medicine composition for treating high insulin polycystic ovary syndrome
InactiveCN1977948AGood curative effectImprove dry mouthPill deliveryGranular deliveryEpimediumAnemarrhena asphodeloides
The present invention relates to a Chinese medicine composition for curing female high-insulin type polycystic ovary syndrome. Said Chinese medicine composition includes 11 Chinese medicinal materials of anemarrhena root, epimedium, tortoise plastron, ophiopogon tuber, lyceum berry, polygonatum root, sea horse, flowery knotweed root and others.
Owner:GREEN VALLEY GROUP CO LTD
Application of compound in preparation of drug for treating type 2 diabetic cardiomyopathy
InactiveCN113304149AMolecular smallIncrease fat solubilityOrganic active ingredientsMetabolism disorderPharmacologyInsulin humulin
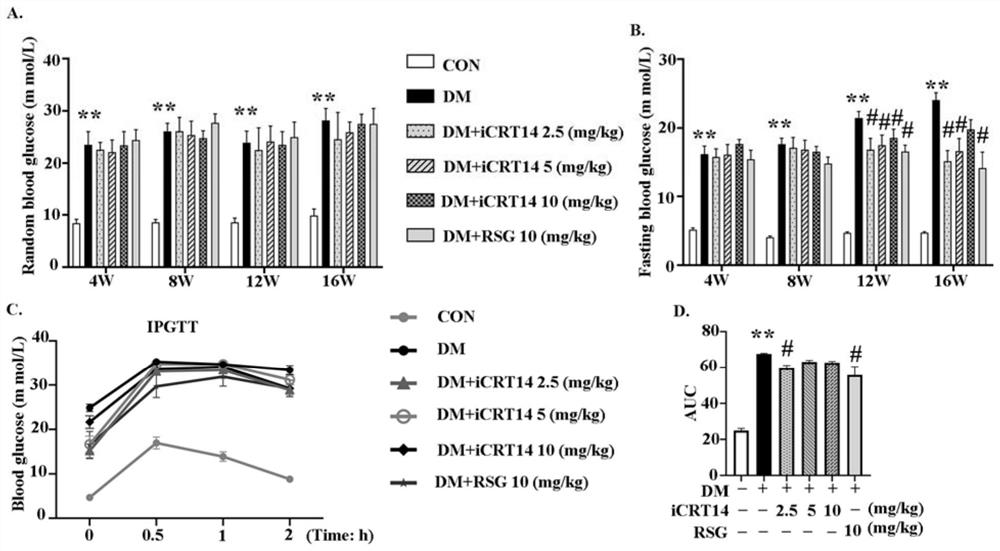
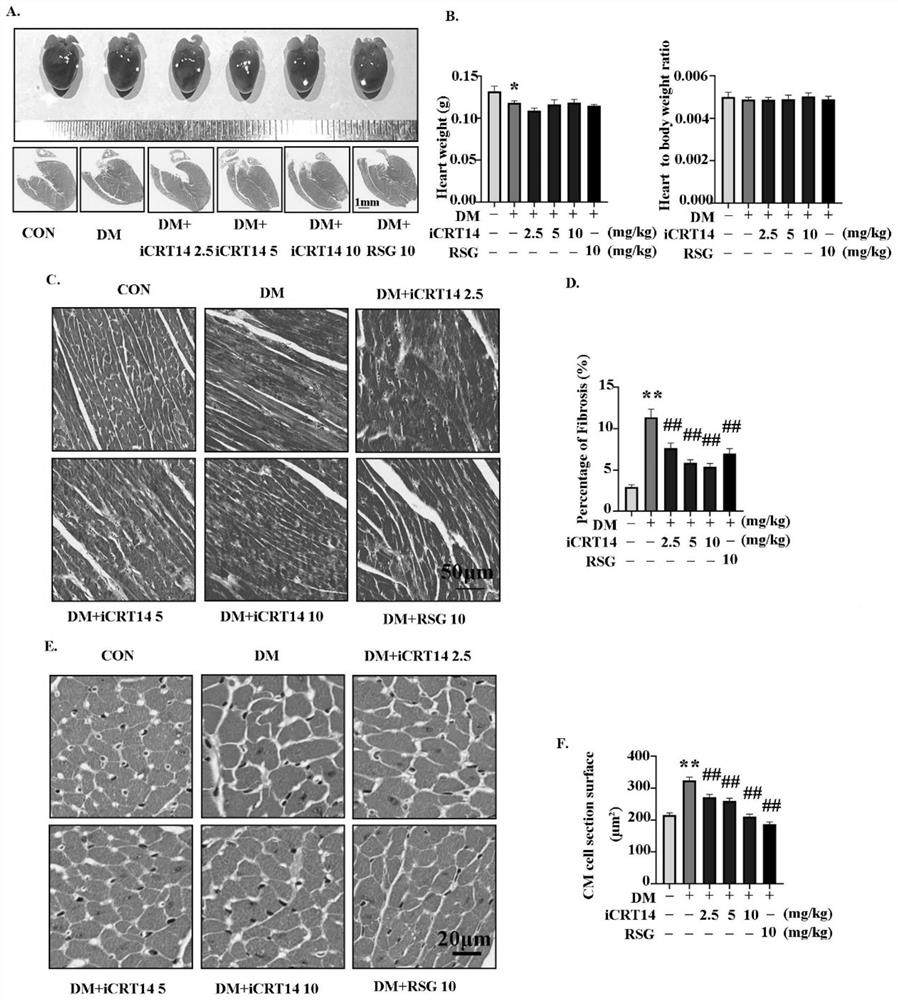
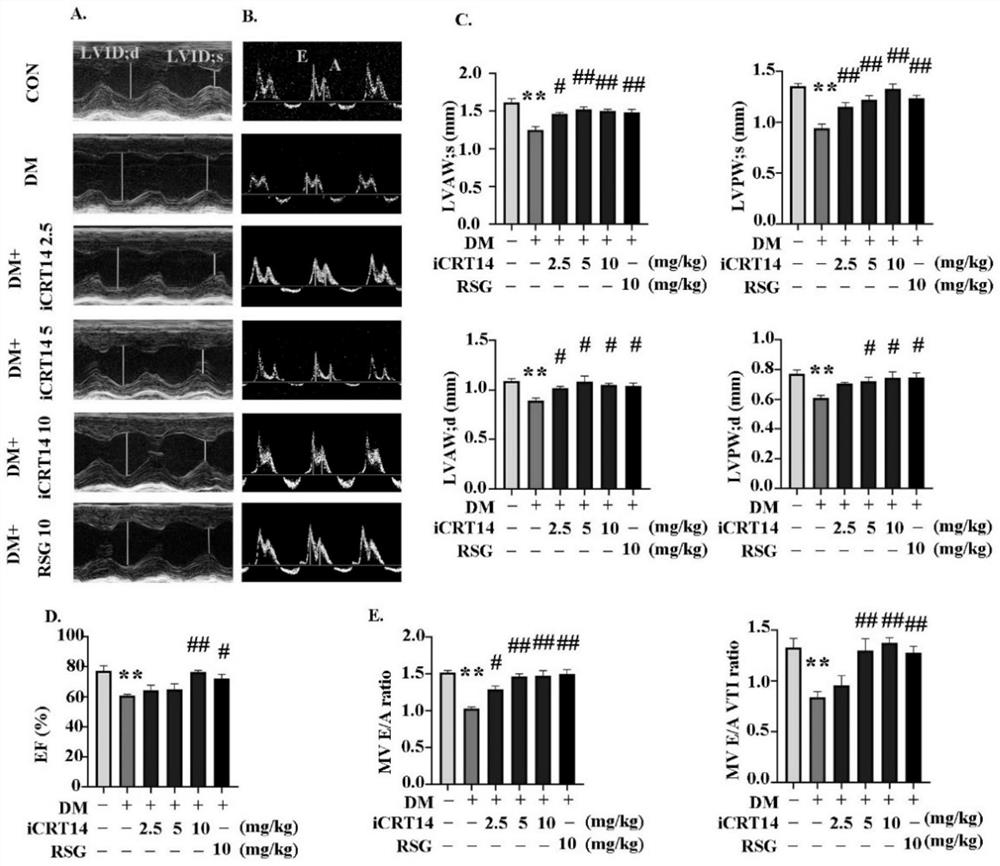
The invention discloses a novel application of iCRT14, and particularly relates to a novel application of iCRT14 in preparation of a drug for treating type 2 diabetic cardiomyopathy. In the research, the inventor of the application finds that: the iCRT14 can reduce the blood sugar of the type 2 diabetic cardiomyopathy, improve myocardial remodeling of the type 2 diabetic cardiomyopathy, improve cardiac contraction and diastolic dysfunction caused by the type 2 diabetic cardiomyopathy and inhibit myocardial cell hypertrophy induced by high glucose and high insulin, and has a good treatment effect on the type 2 diabetic cardiomyopathy. The invention provides a new drug choice for treatment of the type 2 diabetic cardiomyopathy.
Owner:GUANGZHOU MEDICAL UNIV
Traditional Chinese medicine composition for treating diabetes mellitus, and application of traditional Chinese medicine composition
The invention discloses a traditional Chinese medicine composition for treating diabetes mellitus, which is prepared by the following components in parts by weight: 20 parts of radix puerariae, 10 parts of radix polygonati officinalis, 15 parts of snakegourd fruit roots, 10 parts of ophiopogon roots and 10 parts of schisandra chinensis. The traditional Chinese medicine composition is very remarkable in effect, can obviously reduce a fasting blood-glucose level of a T2DM (Type 2 Diabetes Mellitus) insulin resistance rat, can raise an insulin level, and can improve the sensibility of the model rat on insulin.
Owner:李兴阜
Composition for constructing animal model of diabetes mellitus type II
InactiveCN102172261ALow priceShort cycleOrganic active ingredientsMetabolism disorderAcute hyperglycaemiaIn vivo
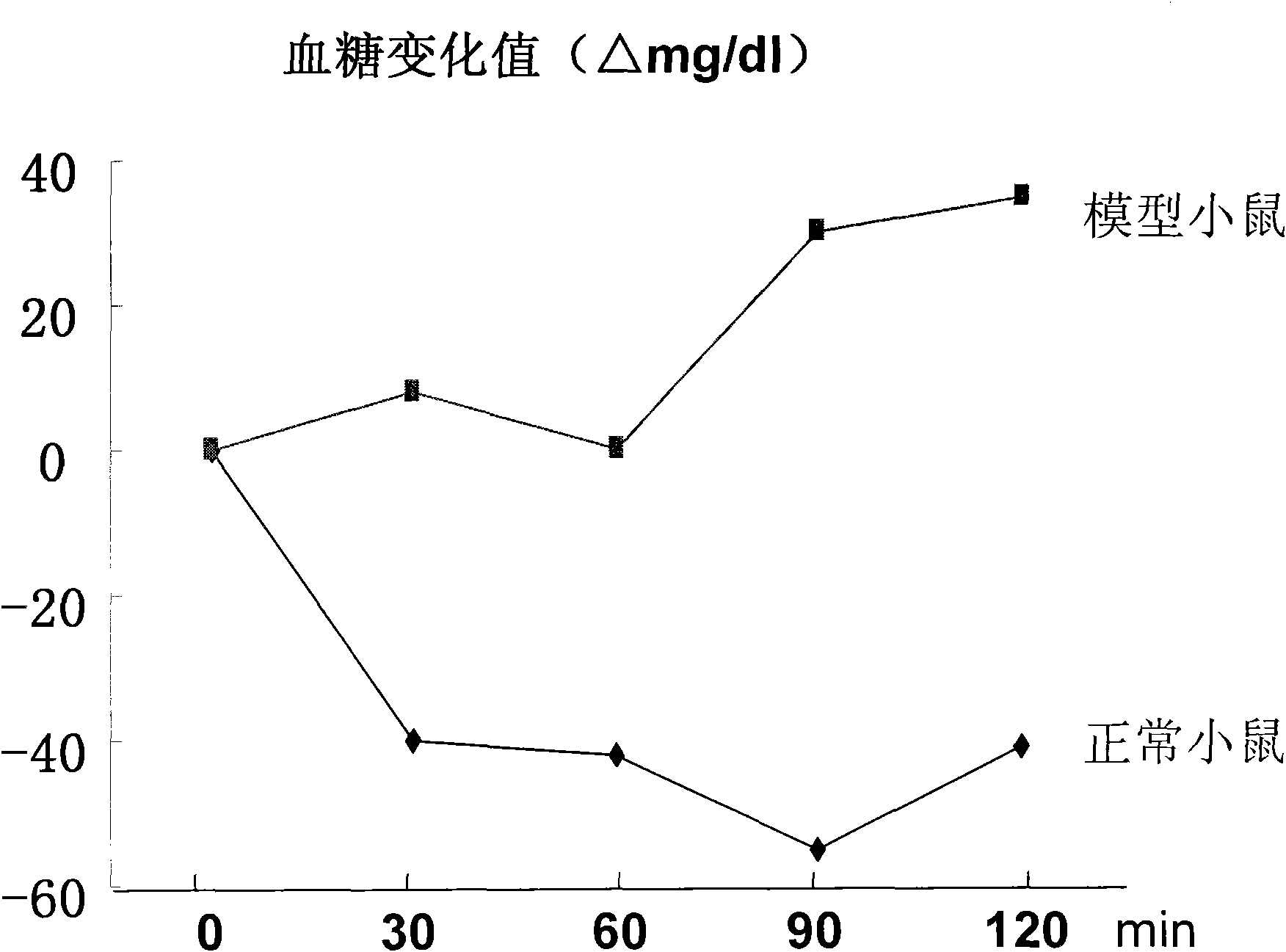
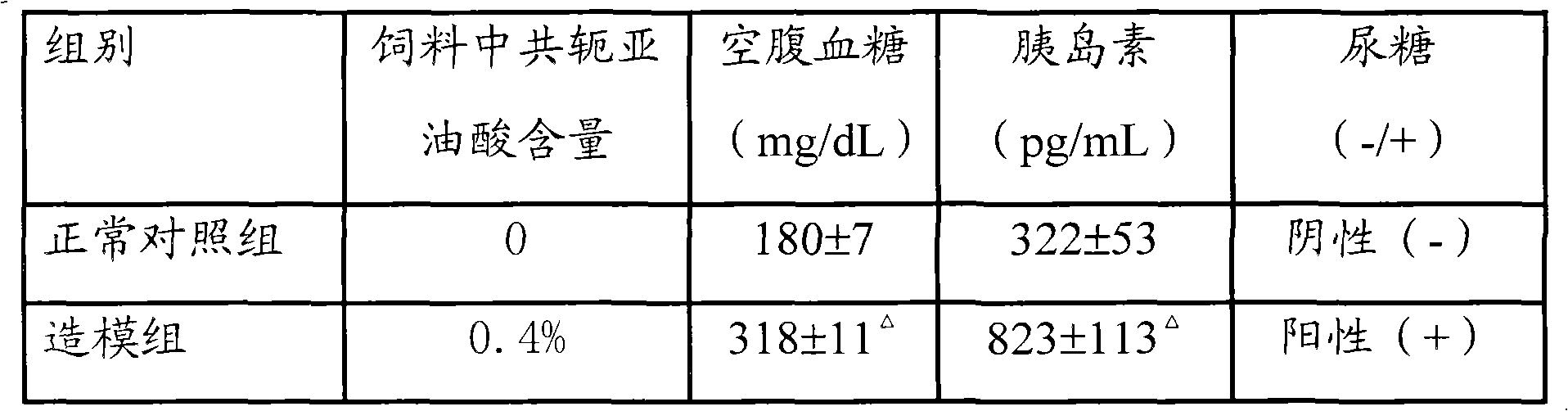
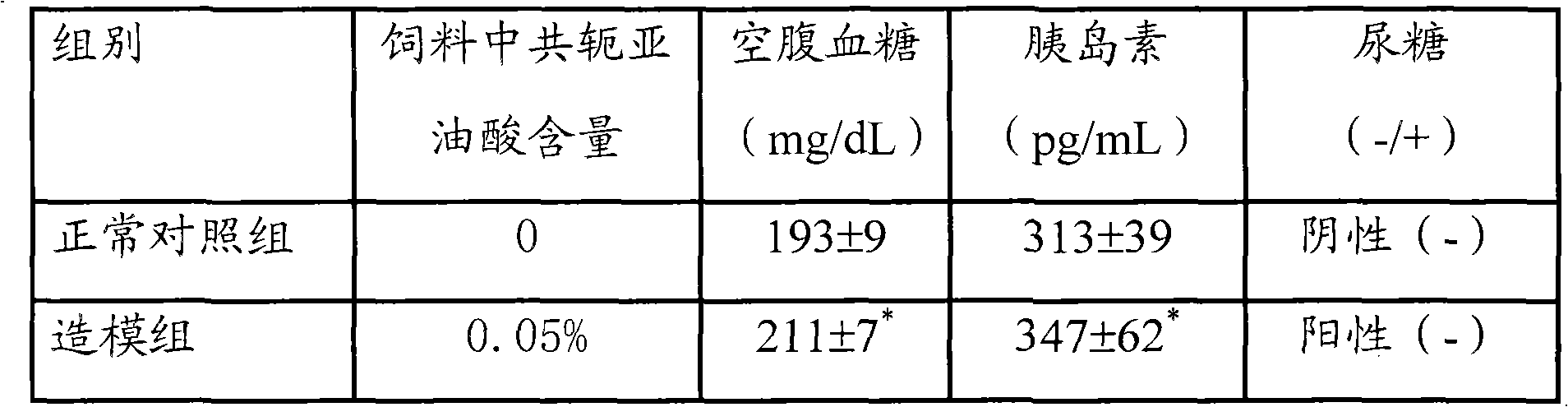
The invention provides a composition for constructing an animal model of diabetes mellitus type II and application of the composition to preparing feeds or drugs for constructing the animal model of the diabetes mellitus type II, wherein the composition comprises a conjugated linoleic acid mixture of which the weight percentage is more than 0.1% relative to the composition; and the animal model of the diabetes mellitus type II is prepared by feeding the composition to the mouse. Because the conjugated linoleic acid mixture or a single conjugated linoleic acid monomer is adopted as an inducer and the mouse is adopted as the animal protomodel, compared with a traditional chemical drug or diet induced animal model of the diabetes mellitus type II, the composition provided by the invention has the advantages of low price, convenience and easiness for raw material obtaining; and because the model establishing time is 4-6 weeks, the period is short, the formed model is characterized by hyperglycemia, high insulin level and in-vivo insulin resistance, the composition accords with basic characteristics of the diabetes mellitus type II and is an ideal composition for constructing the animal model of the diabetes mellitus type II.
Owner:OCEAN UNIV OF CHINA
Medicinal composition for treating hyperinsulinar type polycystic overies and its prepn. method
A Chinese medicine in the form of capsule, tablet or particle for treating high-insulin polycystic ovary syndrome, high-androgen amenorrhoea, etc is prepared from 14 Chinese-medicinal materials including anemarrhena rhizome, epimedium, wolfberry fruit, fleece flower root etc. Its preparing process is also disclosed.
Owner:GREEN VALLEY GROUP CO LTD
Plant solid beverage
InactiveCN110839805AHigh activityImprove the immunityFood ingredient functionsBiotechnologyInsulin Cell
The invention discloses a plant solid beverage and belongs to the technical field of health-care food. The plant solid beverage is produced from, by weight, 10-20 parts of rhizoma dioscoreae, 10-20 parts of rhizoma polygonati, 5-15 parts of folium mori powder, 5-15 parts of pollen pini, 3-8 parts of oat beta-glucan, 3-8 parts of ginseng, 3-8 parts of cortex cinnamomi, 3-8 parts of fructus lycii, 3-8 parts of fructus hippophae, 3-8 parts of radix pueraiae, 3-8 parts of bitter gourd, 3-8 parts of okra, 3-8 parts of glucose-based stevioside and 3-8 parts of mogroside. The compound plant solid beverage has the advantages that the compound plant solid beverage is produced from medicinal and edible traditional Chinese medicine materials and functional auxiliary materials, the mutual influence and interaction of the components allow the compound plant solid beverage to provide comprehensive nutritional support for diabetics, comprehensively supplement nutrients needed by human body cells andincrease insulin cell activity and body resistance, and the plant solid beverage can fast repair damaged blood vessel cells and regulate the intestines and the stomach.
Owner:坤承(北京)健康管理有限公司
Extract, composition, and use of overground part of hedychium coronarium koenig
The present invention is related to the use of an overground part of Hedychium coronarium Koenig in lowering blood glucose, increasing insulin levels and treating and / or preventing diabetes without overly reducing blood glucose in a subject; i.e., not reducing blood glucose in a fasting subject. The present invention also relates to an extract and composition of the overground part of Hedychium coronarium Koenig and its use in lowering blood glucose, increasing insulin levels and treating and / or preventing diabetes.
Owner:DEV CENT FOR BIOTECHNOLOGY
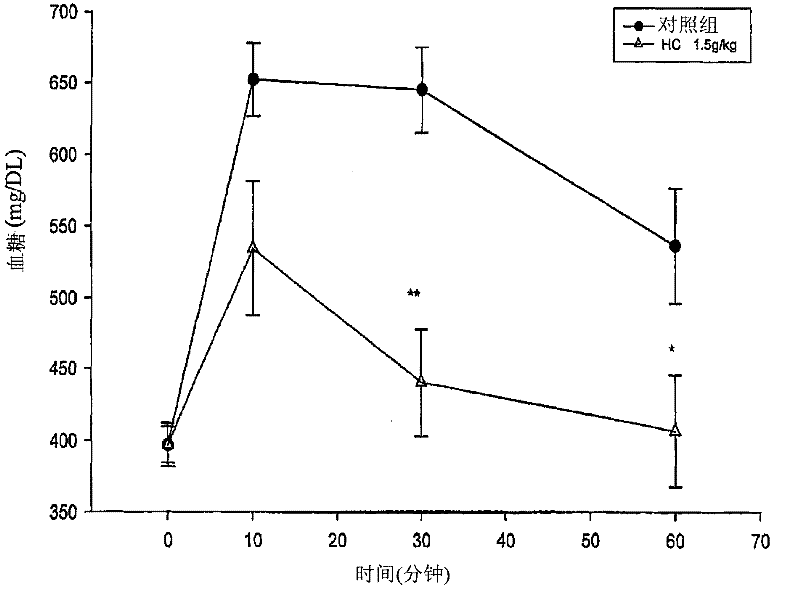
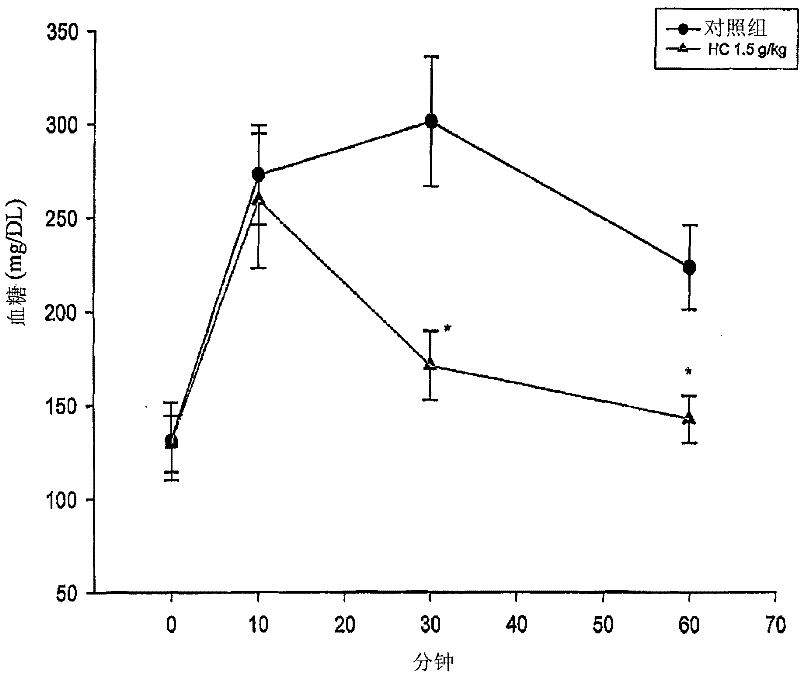
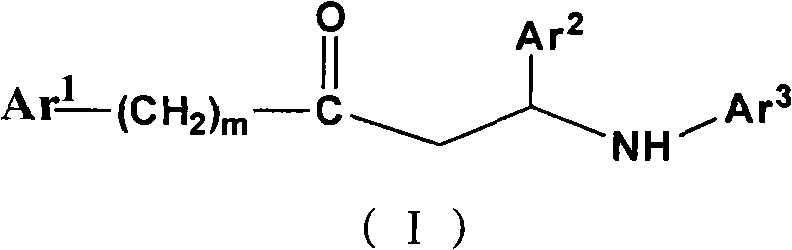
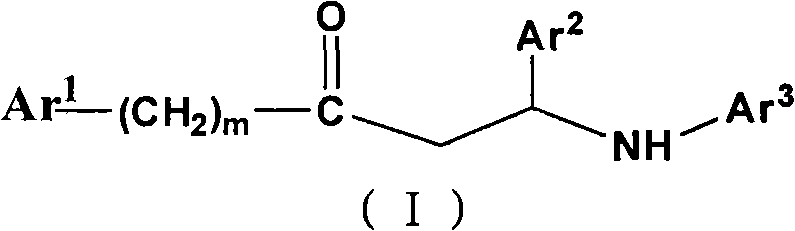
Beta-amino ketones compound with anti-diabetic activity
InactiveCN101538230AGreat development and application valueIncreased sensitivityOrganic chemistryMetabolism disorderPharmaceutical drugPancreatic hormone
The invention discloses a beta-amino ketones compound with anti-diabetic activity, particularly the compound which has the general formula (I) as shown on the right or a pharmaceutically acceptable salt thereof. The invention can increase insulin sensitivity and / or decrease postprandial blood glucose level, and prevent and delay the outbreak and the development of diabetes mellitus and complications thereof, has simple preparation method, low cost and good potential application value and can be prepared to be anti-diabetic medicine for preventing and treating the diabetes mellitus.
Owner:SOUTHWEST UNIV
Method for evaluation of high insulin type obesity gene physique
InactiveCN103602749AImprove detection accuracyGood repeatabilityMicrobiological testing/measurementNutritionLepr gene
The invention discloses a method for evaluation of high insulin type obesity gene physique. The method is characterized by simultaneously detecting and analyzing the SNPs site genotype of individual LEP gene, LEPR gene, IL-6 gene, and ADIPOQ gene to provide high insulin type obesity physique evaluation for all detection subjects. According to the method, the detection subjects can firstly get acquaintance with the gene physique condition of themselves and personal obesity potential cause before considering weight reducing by diet and exercises, so that a most suitable individualized nutrition and exercise weight-loss way can be selected to destroy various obesity gene expression patterns, thus achieving a most effective and healthy weight loss effect.
Owner:上海中优医药高科技股份有限公司
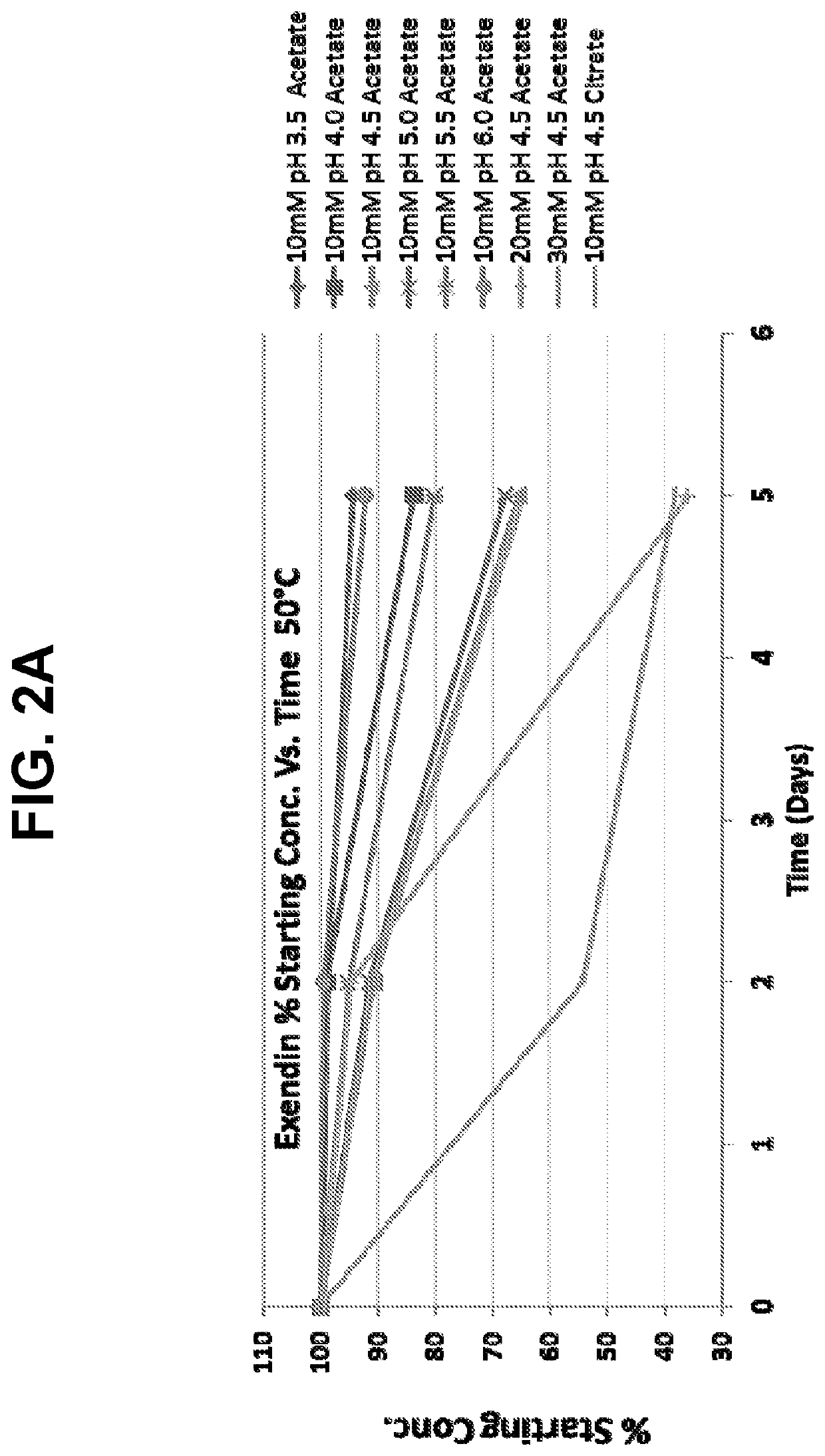
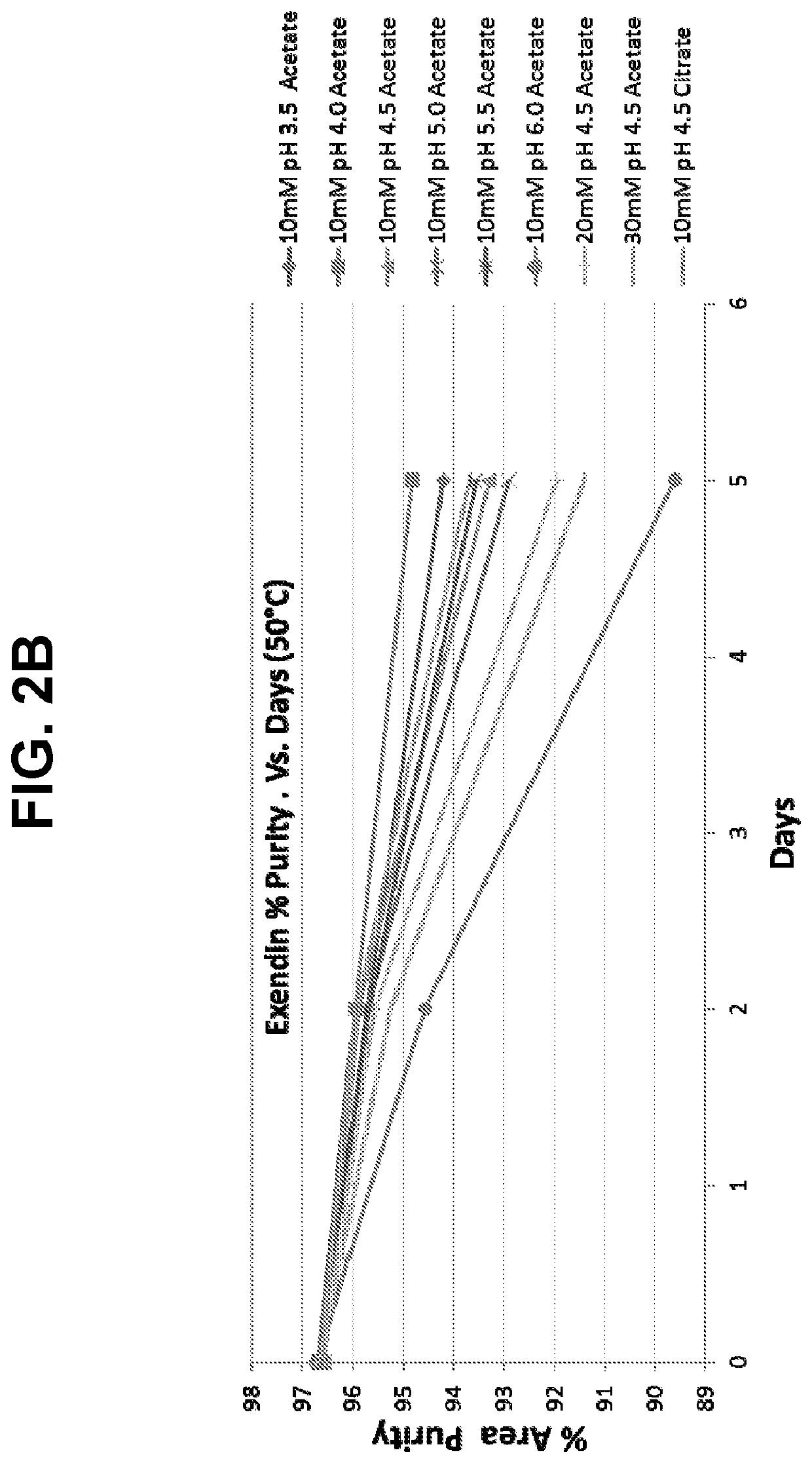
Buffered formulations of exendin (9-39)
Provided herein are liquid pharmaceutical formulations comprising exendin (9-39) or a pharmaceutically acceptable salt thereof and a tonicity modifier in a physiologically acceptable buffer having a pH in the range of about 5 to about 6. In some embodiments, the buffered liquid formulation comprises exendin (9-39) or a pharmaceutically acceptable salt thereof in an acetate buffer or a citrate buffer. Methods of treating or preventing hyperinsulinemic hypoglycemia in a subject comprising administering to the subject the buffered liquid formulation are also provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
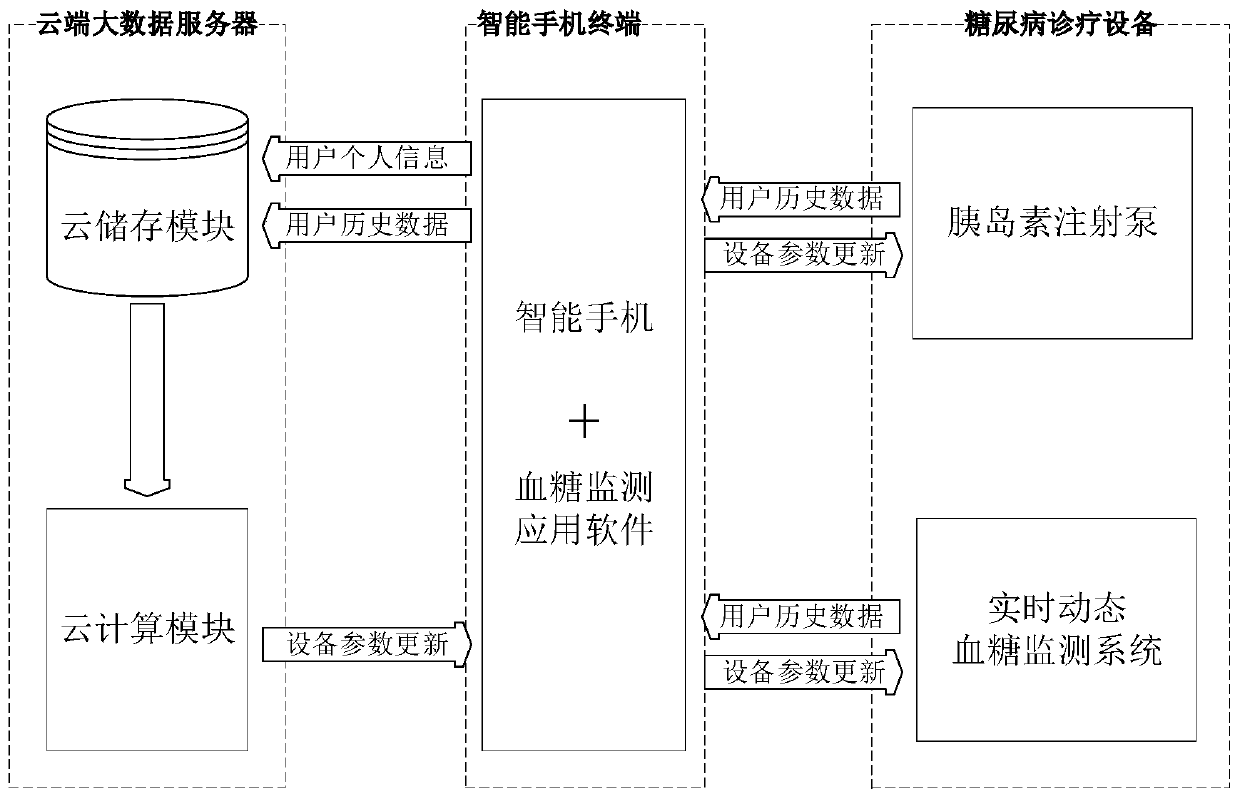
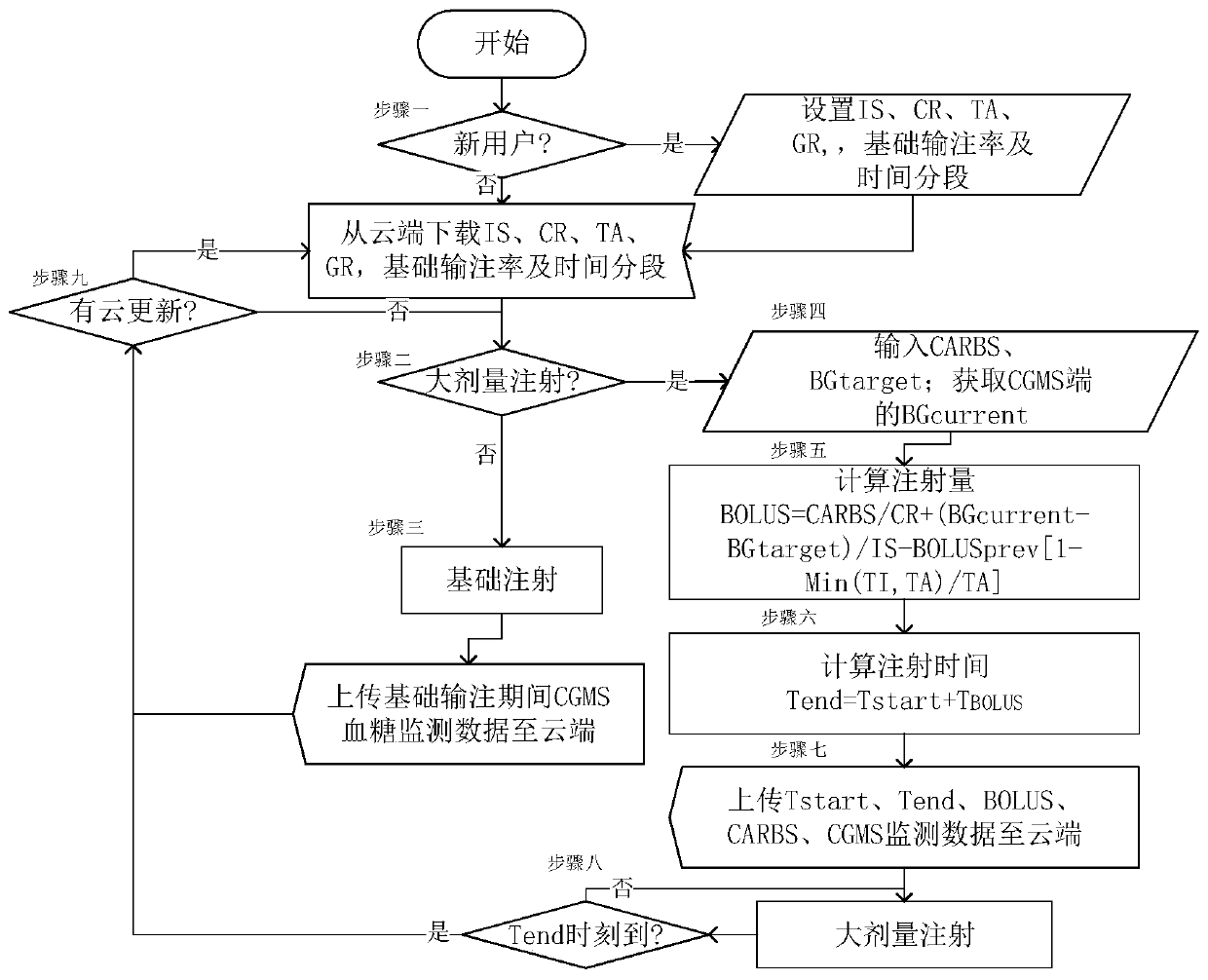
Insulin pump individual configuration optimization system based on cloud big data
ActiveCN107715230BShorten the timeReduce anxietyMedical data miningDrug and medicationsConfiguration optimizationInsulin injection
The invention discloses an insulin pump individualized configuration optimization system and method based on cloud big data, including an insulin pump, a real-time dynamic blood sugar monitoring system, a smart phone, blood sugar monitoring application software installed in the smart phone, and a cloud big data server. The system constructed by the present invention can effectively calculate each user's individual optimal insulin injection volume and injection rate through the user's personal blood glucose measurement history data stored in the cloud, and assist doctors and patients to formulate more effective diabetes treatment plans.
Owner:MICRO TECH MEDICAL HANGZHOU CO LTD
Medicine for preventing and treating cattle and sheep flaccid paralysis diseases and preparation method of medicine
InactiveCN111228298AGood water solubilityGood dispersionHydroxy compound active ingredientsMetabolism disorderDiseasePhysiology
The present invention discloses a medicine for preventing and treating cattle and sheep flaccid paralysis diseases and relates to the technical field of agricultural breeding. Each liter of the medicine contains the following substances: 7 million-11 million units of vitamin A palmitate, 4 million-6 million units of vitamin D3 ethyl acetate, 10-30 g of vitamin E ethyl acetate, 15-25 mg of 25-hydroxycholecalciferol, 100-150 g of calcium gluconate, 500-800 mg of sodium selenite, 50-100 g of butafosfan, 2.5-6 g of adenosine triphosphate, 30-60 g of inositol, 100-150 g of an emulsifier, 20-30 g ofa co-emulsifier, 10-15 g of an antioxidant, 30-50 g of a surfactant and the balance purified water. The medicine can effectively reduce levels of stress hormones in body, increase concentration of insulin, significantly improve liver functions, and promote rapid use of energy while supplementing calcium so as to strengthen muscle functions, enhance vitality, enhance physical fitness, prevent andtreat the cattle and sheep flaccid paralysis diseases, and is high in absorption and utilization rates at the same time.
Owner:开封嘉骏生物科技有限公司
Application of jasminum grandiflorum and jasminum grandiflorum extract in preparation of medicine for treating hyperlipemia, metabolic syndrome or non-alcoholic fatty liver disease
ActiveCN111920853AReduced stamina damageLower triglyceridesMetabolism disorderDigestive systemJasminum grandiflorumPhosphorylation
The invention discloses application of jasminum grandiflorum and jasminum grandiflorum extract in preparation of medicine for treating hyperlipidaemia, metabolic syndrome or non-alcoholic fatty liverdisease, and belongs to the field of medicine. The jasminum grandiflorum and the jasminum grandiflorum extract have the advantages that the obesity caused by high-fat and high-cholesterol food can beobviously reduced; the related glucose tolerance damage is reduced; fat content is greatly reduced, levels of triglyceride and cholesterol in serum and liver of a mouse fed with high fat and high cholesterol can be remarkably reduced, phosphorylation of Akt in an insulin signal channel can be activated, and accordingly insulin sensitivity is improved. The invention develops the medicinal application of jasminum grandiflorum and the extract thereof, and provides a new possibility for treating hyperlipidaemia, metabolic syndrome or non-alcoholic fatty liver disease.
Owner:WUYI UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com